Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy
- PMID: 34337369
- PMCID: PMC8318926
- DOI: 10.1016/j.jhepr.2021.100316
Portal hypertension in cirrhosis: Pathophysiological mechanisms and therapy
Abstract
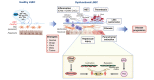
Portal hypertension, defined as increased pressure in the portal vein, develops as a consequence of increased intrahepatic vascular resistance due to the dysregulation of liver sinusoidal endothelial cells (LSECs) and hepatic stellate cells (HSCs), frequently arising from chronic liver diseases. Extrahepatic haemodynamic changes contribute to the aggravation of portal hypertension. The pathogenic complexity of portal hypertension and the unsuccessful translation of preclinical studies have impeded the development of effective therapeutics for patients with cirrhosis, while counteracting hepatic and extrahepatic mechanisms also pose a major obstacle to effective treatment. In this review article, we will discuss the following topics: i) cellular and molecular mechanisms of portal hypertension, focusing on dysregulation of LSECs, HSCs and hepatic microvascular thrombosis, as well as changes in the extrahepatic vasculature, since these are the major contributors to portal hypertension; ii) translational/clinical advances in our knowledge of portal hypertension; and iii) future directions.
Keywords: ACE2, angiogenesis-converting enzyme 2; ACLF, acute-on-chronic liver failure; AT1R, angiotensin II type I receptor; CCL2, chemokine (C-C motif) ligand 2; CCl4, carbon tetrachloride; CLD, chronic liver disease; CSPH, clinically significant portal hypertension; Dll4, delta like canonical Notch ligand 4; ECM, extracellular matrix; EUS, endoscopic ultrasound; FXR; FXR, farnesoid X receptor; HCC, hepatocellular carcinoma; HRS, hepatorenal syndrome; HSC; HSCs, hepatic stellate cells; HVPG, hepatic venous pressure gradient; Hsp90, heat shock protein 90; JAK2, Janus kinase 2; KO, knockout; LSEC; LSEC, liver sinusoidal endothelial cells; MLCP, myosin light-chain phosphatase; NET, neutrophil extracellular trap; NO; NO, nitric oxide; NSBB; NSBBs, non-selective beta blockers; PDE, phosphodiesterase; PDGF, platelet-derived growth factor; PIGF, placental growth factor; PKG, cGMP-dependent protein kinase; Rho-kinase; TIPS; TIPS, transjugular intrahepatic portosystemic shunt; VCAM1, vascular cell adhesion molecule 1; VEGF; VEGF, vascular endothelial growth factor; angiogenesis; eNOS, endothelial nitric oxide synthase; fibrosis; liver stiffness; statins; β-Arr2, β-arrestin 2; β1-AR, β1-adrenergic receptor; β2-AR, β2-adrenergic receptor.
© 2021 The Authors.
Conflict of interest statement
Jonel Trebicka has received speaking and/or consulting fees from Gore, Bayer, Alexion, MSD, Gilead, Intercept, Norgine, Grifols, Versantis, and Martin Pharmaceutical. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures


References
-
- Iwakiri Y., Groszmann R.J. The hyperdynamic circulation of chronic liver diseases: from the patient to the molecule. Hepatology. 2006;43:S121–S131. - PubMed
-
- Hennenberg M., Trebicka J., Sauerbruch T., Heller J. Mechanisms of extrahepatic vasodilation in portal hypertension. Gut. 2008;57:1300–1314. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

