Polymerization-Induced Self-Assembly of Metallo-Polyelectrolyte Block Copolymers
- PMID: 34337427
- PMCID: PMC8324045
- DOI: 10.1002/pola.29439
Polymerization-Induced Self-Assembly of Metallo-Polyelectrolyte Block Copolymers
Abstract
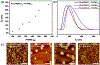
Cobaltocenium-containing polyelectrolyte block copolymer nanoparticles were prepared via polymerization-induced self-assembly (PISA) using aqueous dispersion RAFT polymerization. The cationic steric stabilizer was a macromolecular chain-transfer agent (macro-CTA) based on poly (2-cobaltocenium amidoethyl methacrylate chloride) (PCoAEMACl), and the core-forming block was poly(2-hydroxypropyl methacrylate) (PHPMA). Stable cationic spherical nanoparticles were formed in aqueous solution with low dispersity without adding any salts. The chain extension of macro-CTA with HPMA was efficient and fast. The effects of block copolymer compositions, solid content, charge density, and addition of salts were studied. It was found that the degree of polymerization of both the stabilizer PCoAEMACl and the core-forming PHPMA had a strong influence on the size of nanoparticles.
Keywords: block copolymer; metallopolymer; polyelectrolyte; self-assembly.
Figures





Similar articles
-
Cationic polyelectrolyte-stabilized nanoparticles via RAFT aqueous dispersion polymerization.Langmuir. 2013 Jun 18;29(24):7416-24. doi: 10.1021/la304279y. Epub 2012 Dec 14. Langmuir. 2013. PMID: 23205729
-
Anionic polyelectrolyte-stabilized nanoparticles via RAFT aqueous dispersion polymerization.Langmuir. 2012 Jan 10;28(1):914-22. doi: 10.1021/la203991y. Epub 2011 Dec 12. Langmuir. 2012. PMID: 22115201
-
Synthesis and Nano-object Assembly of Biomimetic Block Copolymers for Catalytic Silver Nanoparticles.Langmuir. 2019 Feb 5;35(5):1346-1356. doi: 10.1021/acs.langmuir.8b01558. Epub 2018 Aug 27. Langmuir. 2019. PMID: 30107737
-
Expanding the Scope of Polymerization-Induced Self-Assembly: Recent Advances and New Horizons.Macromol Rapid Commun. 2021 Dec;42(23):e2100498. doi: 10.1002/marc.202100498. Epub 2021 Aug 27. Macromol Rapid Commun. 2021. PMID: 34418199 Review.
-
Controlling Nanomaterial Size and Shape for Biomedical Applications via Polymerization-Induced Self-Assembly.Macromol Rapid Commun. 2019 Jan;40(2):e1800438. doi: 10.1002/marc.201800438. Epub 2018 Aug 9. Macromol Rapid Commun. 2019. PMID: 30091816 Review.
Cited by
-
Metallo-Polyelectrolytes: Correlating Macromolecular Architectures with Properties and Applications.Trends Chem. 2020 Mar;2(3):227-240. doi: 10.1016/j.trechm.2019.12.004. Epub 2019 Dec 27. Trends Chem. 2020. PMID: 34337370 Free PMC article.
-
Recent advances in metallopolymer-based drug delivery systems.RSC Adv. 2019 Nov 13;9(63):37009-37051. doi: 10.1039/c9ra06678k. eCollection 2019 Nov 11. RSC Adv. 2019. PMID: 35539076 Free PMC article. Review.
-
Antimicrobial cobaltocenium copolymers: tuning amphiphilicity against NDM-1 bacteria.Biomater Sci. 2025 Jul 22;13(15):4232-4244. doi: 10.1039/d5bm00497g. Biomater Sci. 2025. PMID: 40559204 Free PMC article.
References
-
- Mai Y, Eisenberg A, Chem. Soc. Rev 2012, 41, 5969. - PubMed
-
- Won Y-Y, Davis HT, Bates FS, Science 1999, 283, 960. - PubMed
-
- Discher DE, Eisenberg A, Science 2002, 297, 967. - PubMed
-
- Riess G, Prog. Polym. Sci 2003, 28, 1107.
-
- Bae Y, Fukushima S, Harada A, Kataoka K, Angew. Chem. Int. Ed 2003, 42, 4640. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
