Estetrol Cotreatment of Androgen Deprivation Therapy in Infiltrating or Metastatic, Castration-sensitive Prostate Cancer: A Randomized, Double-blind, Phase II Trial (PCombi)
- PMID: 34337526
- PMCID: PMC8317802
- DOI: 10.1016/j.euros.2021.04.005
Estetrol Cotreatment of Androgen Deprivation Therapy in Infiltrating or Metastatic, Castration-sensitive Prostate Cancer: A Randomized, Double-blind, Phase II Trial (PCombi)
Abstract
Background: Androgen deprivation therapy (ADT) for prostate cancer with luteinizing hormone-releasing hormone (LHRH) agonists can be improved.
Objective: To assess safety, the frequency and severity of hot flushes (HFs), bone health, and antitumor effects of high-dose estetrol (HDE4) when combined with ADT.
Design setting and participants: A phase II, double-blind, randomized, placebo-controlled study was conducted in advanced prostate cancer patients requiring ADT (the PCombi study).
Intervention: Patients receiving LHRH agonist treatment were randomized 2:1 to 40 mg HDE4 (n = 41) or placebo (n = 21) cotreatment for 24 wk.
Outcome measurements and statistical analysis: Coprimary endpoints were frequency/severity of HFs and levels of total and free testosterone (T). Secondary endpoints included assessments of bone metabolism (osteocalcin and type I collagen telopeptide [CTX1]), prostate-specific antigen (PSA), and follicle-stimulating hormone (FSH). Efficacy analysis was based on the selected per-protocol (PP) population.
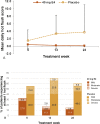
Results and limitations: Of 62 patients included in the study, 57 were suitable for a PP analysis (37 HDE4; 20 placebo). No E4-related serious cardiovascular adverse events occurred at 24 wk. Weekly HFs were reported by 13.5% of patients with HDE4 and 60.0% with placebo (p < 0.001). Daily HFs occurred in 5.9% versus 55%. Bone turnover parameters decreased significantly with HDE4 (p < 0.0001). Total and free T decreased earlier (p < 0.05), and free T was suppressed further (p < 0.05). PSA suppression was more profound and earlier (p < 0.005). FSH levels were suppressed by 98% versus 57% (p < 0.0001). Estrogenic side effects were nipple sensitivity (34%) and gynecomastia (17%).
Conclusions: HDE4 cotreatment of ADT patients with advanced prostate cancer was well tolerated, and no treatment-related cardiovascular adverse events were observed at 24 wk. HFs and bone turnover were substantially reduced. Suppression of free T, PSA, and FSH was more rapid and profound, suggesting enhanced disease control by HDE4 cotreatment. Larger and longer-lasting studies are needed to confirm the results of the study reported here.
Patient summary: Cotreatment of androgen deprivation therapy with high-dose estetrol in advanced prostate cancer patients results in fewer occurrences of hot flushes, bone protection, and other antitumor benefits. Nipple sensitivity and gynecomastia may occur as side effects.
Keywords: Androgen deprivation therapy; Antitumor efficacy; Bone; Cardiovascular safety; Estetrol; Hot flushes; Luteinizing hormone-releasing hormone agonists; PCombi; Prostate cancer.
© 2021 The Authors.
Figures


References
-
- Huggins C., Hodges C.V. Studies on prostatic cancer. I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1941;1:293–297. - PubMed
-
- Presti J.C., Jr Estrogen therapy for prostate carcinoma. JAMA. 1996;275:1153. - PubMed
-
- Turo R., Smolski M., Esler R. Diethylstilboestrol for the treatment of prostate cancer: past, present and future. Scand J Urol. 2014;48:4–14. - PubMed
-
- Matsuo H., Baba Y., Nair R.M., Arimura A., Schally A.V. Structure of the porcine LH- and FSH-releasing hormone. I. The proposed amino acid sequence. Biochem Biophys Res Commun. 1971;43:1334–1339. - PubMed
-
- Coelingh Bennink H.J.T., Zimmerman Y., Verhoeven C. A dose-escalating study with the fetal estrogen estetrol in healthy men. J Clin Endocrinol Metab. 2018;103:3239–3249. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

