Combating information chaos: a case for collaborative clinical guidelines in a pandemic
- PMID: 34337553
- PMCID: PMC8313756
- DOI: 10.1016/j.xcrm.2021.100375
Combating information chaos: a case for collaborative clinical guidelines in a pandemic
Abstract
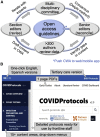
The speed and scale of new information during the COVID-19 pandemic required a new approach toward developing best practices and evidence-based clinical guidance. To address this need, we produced COVIDProtocols.org, a collaborative, evidence-based, digital platform for the development and dissemination of COVID-19 clinical guidelines that has been used by over 500,000 people from 196 countries. We use a Collaborative Writing Application (CWA) to facilitate an expedited expert review process and a web platform that deploys content directly from the CWA to minimize any delays. Over 200 contributors have volunteered to create open creative-commons content that spans over 30 specialties and medical disciplines. Multiple local and national governments, hospitals, and clinics have used the site as a key resource for their own clinical guideline development. COVIDprotocols.org represents a model for efficiently launching open-access clinical guidelines during crisis situations to share expertise and combat misinformation.
Trial registration: ClinicalTrials.gov NCT04389671.
© 2021 The Author(s).
Conflict of interest statement
Covidprotocols.org has been financially supported by USAID, USAID’s Sustaining Technical and Analytic Resources (STAR) project, and the John Doerr Family Foundation. The remaining authors have no disclosures or conflicts of interest relevant to this work. E.Y.K. is a co-investigator in NCT04389671 (Windtree Therapeutics) testing lucinactant (surfactant-like treatment) in COVID-19 patients. E.Y.K. has received unrelated research funding from Bayer, the U.S. National Institutes of Health, the American Heart Association, the American Lung Association, and the American Thoracic Society.
Figures
References
-
- Fraser N., Brierley L., Dey G., Polka J.K., Pálfy M., Coates J.A. Preprinting a pandemic: the role of preprints in the COVID-19 pandemic. bioRxiv. 2020 doi: 10.1101/2020.05.22.111294. - DOI
-
- Horbach S.P.J.M. Pandemic publishing: Medical journals strongly speed up their publication process for COVID-19. Quantitative Science Studies. 2020;1:1056–1067.
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Medical