Plasma cell survival: The intrinsic drivers, migratory signals, and extrinsic regulators
- PMID: 34337772
- PMCID: PMC8387437
- DOI: 10.1111/imr.13013
Plasma cell survival: The intrinsic drivers, migratory signals, and extrinsic regulators
Abstract
Antibody-secreting cells (ASC) are the effectors of protective humoral immunity and the only cell type that produces antibodies or immunoglobulins in mammals. In addition to their formidable capacity to secrete massive quantities of proteins, ASC are terminally differentiated and have unique features to become long-lived plasma cells (LLPC). Upon antigen encounter, B cells are activated through a complex multistep process to undergo fundamental morphological, subcellular, and molecular transformation to become an efficient protein factory with lifelong potential. The ASC survival potential is determined by factors at the time of induction, capacity to migration from induction to survival sites, and ability to mature in the specialized bone marrow microenvironments. In the past decade, considerable progress has been made in identifying factors regulating ASC longevity. Here, we review the intrinsic drivers, trafficking signals, and extrinsic regulators with particular focus on how they impact the survival potential to become a LLPC.
Keywords: antibody-secreting cell; bone marrow; immunoglubulin secretion; long-lived plasma cell; maturation; survival niche.
© 2021 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest
Competing interests: FL is the founder of Micro-Bplex, Inc. FL serves on the scientific board of Be Biopharma, is a recipient of grants from the BMGF and Genentech, Inc. FL has also served as a consultant for Astra Zeneca. IS has consulted for GSK, Pfizer, Kayverna, Johnson & Johnson, Celgene, Bristol Myer Squibb, and Visterra. The other authors declare no conflicts of interest.
Figures



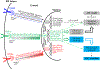
References
-
- Cassese G, Arce S, Hauser AE, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. 2003;171(4):1684–1690. - PubMed
Publication types
MeSH terms
Grants and funding
- R21 AI109601/AI/NIAID NIH HHS/United States
- U19 AI109962/AI/NIAID NIH HHS/United States
- R01 AI121252/AI/NIAID NIH HHS/United States
- P01 AI125180/AI/NIAID NIH HHS/United States
- U01 AI045969/AI/NIAID NIH HHS/United States
- U54 CA260563/CA/NCI NIH HHS/United States
- T32 AI070081/AI/NIAID NIH HHS/United States
- U19 AI110483/AI/NIAID NIH HHS/United States
- U01 AI141993/AI/NIAID NIH HHS/United States
- R37 AI049660/AI/NIAID NIH HHS/United States
- UL1 TR002378/TR/NCATS NIH HHS/United States
- N01 AI050029/AI/NIAID NIH HHS/United States
- R43 TR000023/TR/NCATS NIH HHS/United States
- HHSN266200500030C/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Miscellaneous

