Assessment of the CASP14 assembly predictions
- PMID: 34337786
- PMCID: PMC9109697
- DOI: 10.1002/prot.26199
Assessment of the CASP14 assembly predictions
Abstract
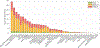
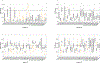
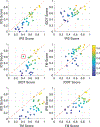
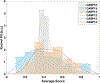
In CASP14, 39 research groups submitted more than 2500 3D models on 22 protein complexes. In general, the community performed well in predicting the fold of the assemblies (for 80% of the targets), although it faced significant challenges in reproducing the native contacts. This is especially the case for the complexes without whole-assembly templates. The leading predictor, BAKER-experimental, used a methodology combining classical techniques (template-based modeling, protein docking) with deep learning-based contact predictions and a fold-and-dock approach. The Venclovas team achieved the runner-up position with template-based modeling and docking. By analyzing the target interfaces, we showed that the complexes with depleted charged contacts or dominating hydrophobic interactions were the most challenging ones to predict. We also demonstrated that if AlphaFold2 predictions were at hand, the interface prediction challenge could be alleviated for most of the targets. All in all, it is evident that new approaches are needed for the accurate prediction of assemblies, which undoubtedly will expand on the significant improvements in the tertiary structure prediction field.
Keywords: CASP; contact prediction; protein assembly; quaternary structure prediction; template-based modeling.
© 2021 Wiley Periodicals LLC.
Conflict of interest statement
Conflict of interest disclosure
The authors declare no conflicts of interest.
Figures
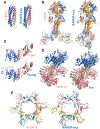
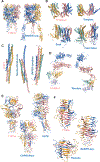

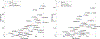
References
-
- Andréll J, Hicks MG, Palmer T, Carpenter EP, Iwata S, & Maher MJ (2009). Crystal structure of the acid-induced arginine decarboxylase from Escherichia coli: reversible decamer assembly controls enzyme activity. Biochemistry, 48(18), 3915–3927. - PubMed

