Advances in chemical probing of protein O-GlcNAc glycosylation: structural role and molecular mechanisms
- PMID: 34338261
- PMCID: PMC8451060
- DOI: 10.1039/d0cs01275k
Advances in chemical probing of protein O-GlcNAc glycosylation: structural role and molecular mechanisms
Abstract
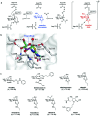
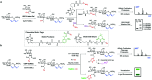
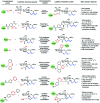
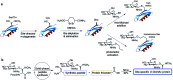
The addition of O-linked-β-D-N-acetylglucosamine (O-GlcNAc) onto serine and threonine residues of nuclear and cytoplasmic proteins is an abundant, unique post-translational modification governing important biological processes. O-GlcNAc dysregulation underlies several metabolic disorders leading to human diseases, including cancer, neurodegeneration and diabetes. This review provides an extensive summary of the recent progress in probing O-GlcNAcylation using mainly chemical methods, with a special focus on discussing mechanistic insights and the structural role of O-GlcNAc at the molecular level. We highlight key aspects of the O-GlcNAc enzymes, including development of OGT and OGA small-molecule inhibitors, and describe a variety of chemoenzymatic and chemical biology approaches for the study of O-GlcNAcylation. Special emphasis is placed on the power of chemistry in the form of synthetic glycopeptide and glycoprotein tools for investigating the site-specific functional consequences of the modification. Finally, we discuss in detail the conformational effects of O-GlcNAc glycosylation on protein structure and stability, relevant O-GlcNAc-mediated protein interactions and its molecular recognition features by biological receptors. Future research in this field will provide novel, more effective chemical strategies and probes for the molecular interrogation of O-GlcNAcylation, elucidating new mechanisms and functional roles of O-GlcNAc with potential therapeutic applications in human health.
Conflict of interest statement
There are no conflicts to declare.
Figures









References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

