New insights into the allosteric effects of CO2 and bicarbonate on crocodilian hemoglobin
- PMID: 34338300
- PMCID: PMC8353160
- DOI: 10.1242/jeb.242615
New insights into the allosteric effects of CO2 and bicarbonate on crocodilian hemoglobin
Abstract
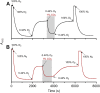
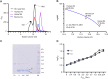
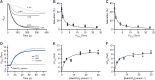
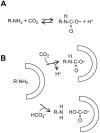
Crocodilians are unique among vertebrates in that their hemoglobin (Hb) O2 binding is allosterically regulated by bicarbonate, which forms in red blood cells upon hydration of CO2. Although known for decades, this remarkable mode of allosteric control has not yet been experimentally verified with direct evidence of bicarbonate binding to crocodilian Hb, probably because of confounding CO2-mediated effects. Here, we provide the first quantitative analysis of the separate allosteric effects of CO2 and bicarbonate on purified Hb of the spectacled caiman (Caiman crocodilus). Using thin-layer gas diffusion chamber and Tucker chamber techniques, we demonstrate that both CO2 and bicarbonate bind to Hb with high affinity and strongly decrease O2 saturation of Hb. We propose that both effectors bind to an unidentified positively charged site containing a reactive amino group in the low-O2 affinity T conformation of Hb. These results provide the first experimental evidence that bicarbonate binds directly to crocodilian Hb and promotes O2 delivery independently of CO2. Using the gas diffusion chamber, we observed similar effects in Hbs of a phylogenetically diverse set of other caiman, alligator and crocodile species, suggesting that the unique mode of allosteric regulation by CO2 and bicarbonate evolved >80-100 million years ago in the common ancestor of crocodilians. Our results show a tight and unusual linkage between O2 and CO2 transport in the blood of crocodilians, where the build-up of erytrocytic CO2 and bicarbonate ions during breath-hold diving or digestion facilitates O2 delivery, while Hb desaturation facilitates CO2 transport as protein-bound CO2 and bicarbonate.
Keywords: Adaptation; Allosteric regulation; Blood; Carbon dioxide; Oxygen.
© 2021. Published by The Company of Biologists Ltd.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures
Similar articles
-
Evolution and molecular basis of a novel allosteric property of crocodilian hemoglobin.Curr Biol. 2023 Jan 9;33(1):98-108.e4. doi: 10.1016/j.cub.2022.11.049. Epub 2022 Dec 21. Curr Biol. 2023. PMID: 36549299 Free PMC article.
-
Lack of conventional oxygen-linked proton and anion binding sites does not impair allosteric regulation of oxygen binding in dwarf caiman hemoglobin.Am J Physiol Regul Integr Comp Physiol. 2013 Aug 1;305(3):R300-12. doi: 10.1152/ajpregu.00014.2013. Epub 2013 May 29. Am J Physiol Regul Integr Comp Physiol. 2013. PMID: 23720132 Free PMC article.
-
Structure and function of crocodilian hemoglobins and allosteric regulation by chloride, ATP, and CO2.Am J Physiol Regul Integr Comp Physiol. 2020 Mar 1;318(3):R657-R667. doi: 10.1152/ajpregu.00342.2019. Epub 2020 Feb 5. Am J Physiol Regul Integr Comp Physiol. 2020. PMID: 32022587 Free PMC article.
-
Red blood cell pH, the Bohr effect, and other oxygenation-linked phenomena in blood O2 and CO2 transport.Acta Physiol Scand. 2004 Nov;182(3):215-27. doi: 10.1111/j.1365-201X.2004.01361.x. Acta Physiol Scand. 2004. PMID: 15491402 Review.
-
A unique mode of tissue oxygenation and the adaptive radiation of teleost fishes.J Exp Biol. 2014 Apr 15;217(Pt 8):1205-14. doi: 10.1242/jeb.093526. J Exp Biol. 2014. PMID: 24744420 Review.
Cited by
-
Changes in hemoglobin function and isoform expression during embryonic development in the American alligator, Alligator mississippiensis.Am J Physiol Regul Integr Comp Physiol. 2021 Dec 1;321(6):R869-R878. doi: 10.1152/ajpregu.00047.2021. Epub 2021 Oct 27. Am J Physiol Regul Integr Comp Physiol. 2021. PMID: 34704846 Free PMC article.
-
Evolution and molecular basis of a novel allosteric property of crocodilian hemoglobin.Curr Biol. 2023 Jan 9;33(1):98-108.e4. doi: 10.1016/j.cub.2022.11.049. Epub 2022 Dec 21. Curr Biol. 2023. PMID: 36549299 Free PMC article.
-
Bicarbonate signalling via G protein-coupled receptor regulates ischaemia-reperfusion injury.Nat Commun. 2024 Feb 27;15(1):1530. doi: 10.1038/s41467-024-45579-3. Nat Commun. 2024. PMID: 38413581 Free PMC article.
-
The unique allosteric property of crocodilian haemoglobin elucidated by cryo-EM.Nat Commun. 2024 Aug 2;15(1):6505. doi: 10.1038/s41467-024-49947-x. Nat Commun. 2024. PMID: 39090102 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

