Plasma proteins, cognitive decline, and 20-year risk of dementia in the Whitehall II and Atherosclerosis Risk in Communities studies
- PMID: 34338426
- PMCID: PMC9292245
- DOI: 10.1002/alz.12419
Plasma proteins, cognitive decline, and 20-year risk of dementia in the Whitehall II and Atherosclerosis Risk in Communities studies
Abstract
Introduction: Plasma proteins affect biological processes and are common drug targets but their role in the development of Alzheimer's disease and related dementias remains unclear. We examined associations between 4953 plasma proteins and cognitive decline and risk of dementia in two cohort studies with 20-year follow-ups.
Methods: In the Whitehall II prospective cohort study proteins were measured using SOMAscan technology. Cognitive performance was tested five times over 20 years. Linkage to electronic health records identified incident dementia. The results were replicated in the Atherosclerosis Risk in Communities (ARIC) study.
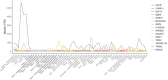
Results: Fifteen non-amyloid/non-tau-related proteins were associated with cognitive decline and dementia, were consistently identified in both cohorts, and were not explained by known dementia risk factors. Levels of six of the proteins are modifiable by currently approved medications for other conditions.
Discussion: This study identified several plasma proteins in dementia-free people that are associated with long-term risk of cognitive decline and dementia.
Keywords: cognitive decline; cohort study; dementia; longitudinal study; proteomics.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
In this academic–industry partnership project, academic collaborators generated the hypothesis and study design and SomaLogic, Inc. provided expertise in plasma proteins and funded the SOMAscan assays. Joni V. Lindbohm and Pyry N. Sipila have received personal lecture fees from the University of Helsinki. Nina Mars reports no conflicts of interest. Keenan A. Walker reports personal lecture fee from the Boston University Medical Center and holds the Programming Chair at the National Academy of Neuropsychology. Eric J. Brunner reports Osaka University research capacity building grant paid to employer. Archana Singh‐Manoux reports no conflicts of interest. Gill Livingston reports no conflicts of interest. Kalle Saksela reports no conflicts of interest. Jane E. Ferrie reports no conflicts of interest. Ruth C. Lovering reports personal lecture fees from the University College London, funding from a COST action grant, and is a member of the executive committee for the International Society of Biocuration (a voluntary role and no payment has been or will be made). Stephen A. Williams is employee of SomaLogic Inc., which has a commercial interest in the results and co‐inventor on multiple patents for specific proteomic models of disease. None of these patents relate to dementia (the topic of the manuscript). Aroon D. Hingorani reports no conflicts of interest. Rebecca F. Gottesman reports personal lecture fees for speaking at University of Michigan grand rounds, University of Alabama McKnight lecture, and the American College of Cardiology conference. Rebecca F. Gottesman is the secretary for the American Neurological Association. Henrik Zetterberg reports that he has served on the scientific advisory boards for Denali, Roche Diagnostics, Wave, Samumed, Siemens Healthineers, Pinteon Therapeutics, and CogRx; has given lectures in symposia sponsored by Fujirebio, Alzecure, and Biogen; and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program. Henrik Zetterberg is also the chair of the Alzheimer's Association Global Biomarker Standardization Consortium and the Alzheimer's Association Biolfluid‐Based Biomarker Professional Interest Area. Mika Kivimaki reports no conflicts of interest.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
- UL1 RR025005/RR/NCRR NIH HHS/United States
- U01 HL096917/HL/NHLBI NIH HHS/United States
- 221854/Z/20/Z/WT_/Wellcome Trust/United Kingdom
- R01 HL086694/HL/NHLBI NIH HHS/United States
- U01 HL096902/HL/NHLBI NIH HHS/United States
- U01 HG004402/HG/NHGRI NIH HHS/United States
- RG/16/11/32334/BHF_/British Heart Foundation/United Kingdom
- HHSN268201700001I/HL/NHLBI NIH HHS/United States
- HHSN268201700004I/HL/NHLBI NIH HHS/United States
- U01 HL096814/HL/NHLBI NIH HHS/United States
- R56 AG056477/AG/NIA NIH HHS/United States
- R01 HL087641/HL/NHLBI NIH HHS/United States
- HHSN268201700003I/HL/NHLBI NIH HHS/United States
- R01 AG056477/AG/NIA NIH HHS/United States
- U01 HL096812/HL/NHLBI NIH HHS/United States
- RG/13/2/30098/BHF_/British Heart Foundation/United Kingdom
- MR/R024227/1/MRC_/Medical Research Council/United Kingdom
- K23 AG064122/AG/NIA NIH HHS/United States
- HHSN268201700002C/HL/NHLBI NIH HHS/United States
- RF1 AG062553/AG/NIA NIH HHS/United States
- HHSN268201700005C/HL/NHLBI NIH HHS/United States
- HHSN268201700001C/HL/NHLBI NIH HHS/United States
- MR/S011676/1/MRC_/Medical Research Council/United Kingdom
- HHSN268201700003C/HL/NHLBI NIH HHS/United States
- U01 HL096899/HL/NHLBI NIH HHS/United States
- HHSN268201700004C/HL/NHLBI NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- K24 AG052573/AG/NIA NIH HHS/United States
- HHSN268201700002I/HL/NHLBI NIH HHS/United States
- HHSN268201700005I/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

