Oocyte quality and aging
- PMID: 34338482
- PMCID: PMC8769179
- DOI: 10.5935/1518-0557.20210026
Oocyte quality and aging
Abstract
It is well known that female reproduction ability decreases during the forth decade of life due to age-related changes in oocyte quality and quantity; although the number of women trying to conceive has today increased remarkably between the ages of 36 to 44. The causes of reproductive aging and physiological aspects of this phenomenon are still elusive. With increase in the women's age, during Assisted Reproductive Technologies (ART) we have perceived a significant decline in the number and quality of retrieved oocytes, as well as in ovarian follicle reserves. This is because of increased aneuploidy due to factors such as spindle apparatus disruption; oxidative stress and mitochondrial damage. The aim of this review paper is to study data on the potential role of the aging process impacting oocyte quality and female reproductive ability. We present the current evidence that show the decreased oocyte quality with age, related to reductions in female reproductive outcome. The aging process is complicated and it is caused by many factors that control cellular and organism life span. Although the factors responsible for reduced oocyte quality remain unknown, the present review focuses on the potential role of ovarian follicle environment, oocyte structure and its organelles. To find a way to optimize oocyte quality and ameliorate clinical outcomes for women with aging-related causes of infertility.
Keywords: aging; female infertility; oocyte quality; ovary.
Conflict of interest statement
The authors declare that there is no conflict of interests.
Figures
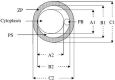


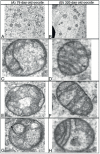
References
-
- Absalan F, Saremy S, Mansori E, Taheri Moghadam M, Eftekhari Moghadam AR, Ghanavati R. Effects of Mono-(2-Ethylhexyl) Phthalate and Di-(2-Ethylhexyl) Phthalate Administrations on Oocyte Meiotic Maturation, Apoptosis and Gene Quantification in Mouse Model. Cell J. 2017;18:503–513. doi: 10.22074/cellj.2016.4717. - DOI - PMC - PubMed
-
- Baird DT, Collins J, Egozcue J, Evers LH, Gianaroli L, Leridon H, Sunde A, Templeton A, Van Steirteghem A, Cohen J, Crosignani PG, Devroey P, Diedrich K, Fauser BC, Fraser L, Glasier A, Liebaers I, Mautone G, Penney G, Tarlatzis B, ESHRE Capri Workshop Group Fertility and ageing. Hum Reprod Update. 2005;11:261–276. doi: 10.1093/humupd/dmi006. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

