Depression Outcomes Among Patients Treated With Fluoxetine for Stroke Recovery: The AFFINITY Randomized Clinical Trial
- PMID: 34338714
- PMCID: PMC8329781
- DOI: 10.1001/jamaneurol.2021.2418
Depression Outcomes Among Patients Treated With Fluoxetine for Stroke Recovery: The AFFINITY Randomized Clinical Trial
Abstract
Importance: One in 3 adults experiences clinically significant symptoms of depression during the first year after a stroke, but evidence to support the use of antidepressants in this population remains scant.
Objective: To investigate whether daily treatment with 20 mg of fluoxetine hydrochloride reduces the proportion of people affected by clinically significant symptoms of depression after stroke.
Design, setting, and participants: In this secondary analysis of the Assessment of Fluoxetine in Stroke Recovery parallel-group, randomized (1:1 assignment), double-blind, placebo-controlled clinical trial, 1221 participants in Australia, New Zealand, and Vietnam were recruited between January 11, 2013, and June 30, 2019, and were followed up for 6 months. Adults aged 18 years or older were recruited 2 to 15 days after experiencing a stroke associated with modified Rankin Scale score of 1 or higher.
Interventions: Fluoxetine hydrochloride, 20 mg, or matched placebo daily for 26 weeks.
Main outcomes and measures: A 9-item Patient Health Questionnaire (PHQ-9) score of 9 or lower was a prespecified secondary outcome of the trial. Assessments were completed at baseline and at 4, 12, and 26 weeks. Other outcomes of interest included participant-reported clinician diagnosis of depression, prescription of a nontrial antidepressant, or nonpharmacologic treatment of depression. Analysis was on an intention-to-treat basis.
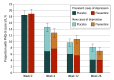
Results: A total of 607 participants (378 men [62.3%]; mean [SD] age, 64.3 [12.2] years) were randomly assigned treatment with placebo, and 614 participants (397 men [64.7%]; mean [SD] age, 63.4 [12.4] years) were randomly assigned treatment with 20 mg of fluoxetine hydrochloride daily. The groups were balanced for demographic and clinical measures. At baseline, 112 patients (18.5%) in the placebo group and 116 patients (18.9%) in the fluoxetine group had PHQ-9 scores of 9 or higher. During follow-up, 126 of 596 participants (21.1%) treated with placebo and 121 of 598 participants (20.2%) treated with fluoxetine had PHQ-9 scores of 9 or higher (P = .70). A similar proportion of participants with PHQ-9 scores less than 9 at baseline who were treated with fluoxetine hydrochloride and placebo developed PHQ-9 scores of 9 or higher during the trial (placebo, 72 of 488 [14.8%]; and fluoxetine, 63 of 485 [13.0%]; P = .43). A slightly higher number of participants in the placebo group than in the fluoxetine group had a participant-reported clinician diagnosis of depression (42 of 602 [7.0%] vs 26 of 601 [4.3%]; P = .05). By week 26, 14 participants (2.3%) in the placebo group and 12 participants (1.9%) in the fluoxetine group had died (P = .67).
Conclusions and relevance: Routine daily treatment with 20 mg of fluoxetine did not decrease the proportion of people affected by clinically significant symptoms of depression after a stroke, nor did it affect the proportion of people prescribed an antidepressant or receiving nonpharmacologic treatments compared with placebo.
Trial registration: http://anzctr.org.au Identifier: ACTRN12611000774921.
Conflict of interest statement
Figures

Comment in
-
Poststroke Selective Serotonin Reuptake Inhibitors-Do They Work for Anything?JAMA Neurol. 2021 Sep 1;78(9):1053-1054. doi: 10.1001/jamaneurol.2021.1907. JAMA Neurol. 2021. PMID: 34338719 No abstract available.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

