Direct to Angiography Suite Without Stopping for Computed Tomography Imaging for Patients With Acute Stroke: A Randomized Clinical Trial
- PMID: 34338742
- PMCID: PMC8329790
- DOI: 10.1001/jamaneurol.2021.2385
Direct to Angiography Suite Without Stopping for Computed Tomography Imaging for Patients With Acute Stroke: A Randomized Clinical Trial
Abstract
Importance: Direct transfer to angiography suite (DTAS) for patients with suspected large vessel occlusion (LVO) stroke has been described as an effective and safe measure to reduce workflow time in endovascular treatment (EVT). However, it is unknown whether DTAS improves long-term functional outcomes.
Objective: To explore the effect of DTAS on clinical outcomes among patients with LVO stroke in a randomized clinical trial.
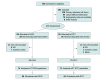
Design, setting, and participants: The study was an investigator-initiated, single-center, evaluator-blinded randomized clinical trial. Of 466 consecutive patients with acute stroke screened, 174 with suspected LVO acute stroke within 6 hours of symptom onset were included. Enrollment took place from September 2018 to November 2020 and was stopped after a preplanned interim analysis. Final follow-up was in February 2021.
Interventions: Patients were randomly assigned (1:1) to follow either DTAS (89 patients) or conventional workflow (85 patients received direct transfer to computed tomographic imaging, with usual imaging performed and EVT indication decided) to assess the indication of EVT. Patients were stratified according to their having been transferred from a primary center vs having a direct admission.
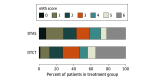
Main outcomes and measures: The primary outcome was a shift analysis assessing the distribution of the 90-day 7-category (from 0 [no symptoms] to 6 [death]) modified Rankin Scale (mRS) score among patients with LVO whether or not they received EVT (modified intention-to-treat population) assessed by blinded external evaluators. Secondary outcomes included rate of EVT and door-to-arterial puncture time. Safety outcomes included 90-day mortality and rates of symptomatic intracranial hemorrhage.
Results: In total, 174 patients were included, with a mean (SD) age of 73.4 (12.6) years (range, 19-95 years), and 78 patients (44.8%) were women. Their mean (SD) onset-to-door time was 228.0 (117.9) minutes, and their median admission National Institutes of Health Stroke Scale score was 18 (interquartile range [IQR], 14-21). In the modified intention-to-treat population, EVT was performed for all 74 patients in the DTAS group and for 64 patients (87.7%) in the conventional workflow group (P = .002). The DTAS protocol decreased the median door-to-arterial puncture time (18 minutes [IQR, 15-24 minutes] vs 42 minutes [IQR, 35-51 minutes]; P < .001) and door-to-reperfusion time (57 minutes [IQR, 43-77 minutes] vs 84 minutes [IQR, 63-117 minutes]; P < .001). The DTAS protocol decreased the severity of disability across the range of the mRS (adjusted common odds ratio, 2.2; 95% CI, 1.2-4.1; P = .009). Safety variables were comparable between groups.
Conclusions and relevance: For patients with LVO admitted within 6 hours after symptom onset, this randomized clinical trial found that, compared with conventional workflow, the use of DTAS increased the odds of patients undergoing EVT, decreased hospital workflow time, and improved clinical outcome.
Trial registration: ClinicalTrials.gov Identifier: NCT04001738.
Conflict of interest statement
Figures
Comment in
-
Disentangling Workflow Paradigms and Treatment Decision-Making in Acute Ischemic Stroke-Reply.JAMA Neurol. 2022 Mar 1;79(3):312. doi: 10.1001/jamaneurol.2021.5346. JAMA Neurol. 2022. PMID: 35129613 No abstract available.
-
Disentangling Workflow Paradigms and Treatment Decision-making in Acute Ischemic Stroke.JAMA Neurol. 2022 Mar 1;79(3):311-312. doi: 10.1001/jamaneurol.2021.5343. JAMA Neurol. 2022. PMID: 35129619 No abstract available.
References
-
- Powers WJ, Rabinstein AA, Ackerson T, et al. . Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi:10.1161/STR.0000000000000211 - DOI - PubMed

