Small molecule inhibitors of mammalian GSK-3β promote in vitro plant cell reprogramming and somatic embryogenesis in crop and forest species
- PMID: 34338766
- PMCID: PMC8664590
- DOI: 10.1093/jxb/erab365
Small molecule inhibitors of mammalian GSK-3β promote in vitro plant cell reprogramming and somatic embryogenesis in crop and forest species
Abstract
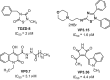
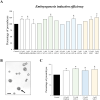
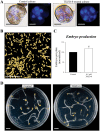
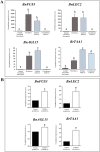
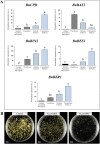
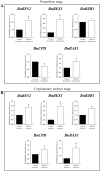
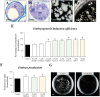
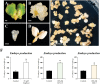
Plant in vitro regeneration systems, such as somatic embryogenesis, are essential in breeding; they permit propagation of elite genotypes, production of doubled-haploids, and regeneration of whole plants from gene editing or transformation events. However, in many crop and forest species, somatic embryogenesis is highly inefficient. We report a new strategy to improve in vitro embryogenesis using synthetic small molecule inhibitors of mammalian glycogen synthase kinase 3β (GSK-3β), never used in plants. These inhibitors increased in vitro embryo production in three different systems and species, microspore embryogenesis of Brassica napus and Hordeum vulgare, and somatic embryogenesis of Quercus suber. TDZD-8, a representative compound of the molecules tested, inhibited GSK-3 activity in microspore cultures, and increased expression of embryogenesis genes FUS3, LEC2, and AGL15. Plant GSK-3 kinase BIN2 is a master regulator of brassinosteroid (BR) signalling. During microspore embryogenesis, BR biosynthesis and signalling genes CPD, GSK-3-BIN2, BES1, and BZR1 were up-regulated and the BAS1 catabolic gene was repressed, indicating activation of the BR pathway. TDZD-8 increased expression of BR signalling elements, mimicking BR effects. The findings support that the small molecule inhibitors promoted somatic embryogenesis by activating the BR pathway, opening up the way for new strategies using GSK-3β inhibitors that could be extended to other species.
Keywords: Barley; brassinosteroids; cell reprogramming; cork oak; glycogen synthase kinase; microspore embryogenesis; rapeseed; small molecule inhibitors; somatic embryogenesis.
© The Author(s) 2021. Published by Oxford University Press on behalf of the Society for Experimental Biology.
Figures


Comment in
-
Small molecules mediate cellular reprogramming across two kingdoms.J Exp Bot. 2021 Dec 4;72(22):7645-7647. doi: 10.1093/jxb/erab493. J Exp Bot. 2021. PMID: 34865113
References
-
- Baki A, Bielik A, Molnár L, Szendrei G, Keserü GM. 2007. A high throughput luminescent assay for glycogen synthase kinase-3beta inhibitors. Assay and Drug Development Technologies 5, 75–83. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

