Hypersensitivity Reactions to Platinum Agents and Taxanes
- PMID: 34338975
- PMCID: PMC9156473
- DOI: 10.1007/s12016-021-08877-y
Hypersensitivity Reactions to Platinum Agents and Taxanes
Abstract
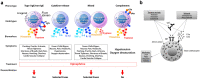
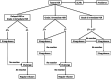
Hypersensitivity reactions (HSRs) to chemotherapy agents can present a serious challenge to treating patients with preferred or first-line therapies. Allergic reactions through an immunologic mechanism have been established for platinum and taxane agents, which are used to treat a wide variety of cancers including gynecologic cancers. Platin HSRs typically occur after multiple cycles of chemotherapy, reflecting the development of drug IgE sensitization, while taxane HSRs often occur on first or second exposure. Despite observed differences between platin and taxane HSRs, drug desensitization has been an effective method to reintroduce both chemotherapeutic agents safely. Skin testing is the primary diagnostic tool used to risk-stratify patients after initial HSRs, with more widespread use for platinum agents than taxanes. Different practices exist around the use of skin testing, drug challenge, and choice of desensitization protocol. Here, we review the epidemiology, mechanism, and clinical presentation of HSRs to platinum and taxane agents, as well as key controversies in their evaluation and management.
Keywords: Chemotherapy; Desensitization; Drug allergy; Hypersensitivity; Platinum agent; Taxane.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures