Chemo-Biological Upcycling of Poly(ethylene terephthalate) to Multifunctional Coating Materials
- PMID: 34339110
- PMCID: PMC8519047
- DOI: 10.1002/cssc.202100909
Chemo-Biological Upcycling of Poly(ethylene terephthalate) to Multifunctional Coating Materials
Abstract
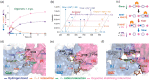
Chemo-biological upcycling of poly(ethylene terephthalate) (PET) developed in this study includes the following key steps: chemo-enzymatic PET depolymerization, biotransformation of terephthalic acid (TPA) into catechol, and its application as a coating agent. Monomeric units were first produced through PET glycolysis into bis(2-hydroxyethyl) terephthalate (BHET), mono(2-hydroxyethyl) terephthalate (MHET), and PET oligomers, and enzymatic hydrolysis of these glycolyzed products using Bacillus subtilis esterase (Bs2Est). Bs2Est efficiently hydrolyzed glycolyzed products into TPA as a key enzyme for chemo-enzymatic depolymerization. Furthermore, catechol solution produced from TPA via a whole-cell biotransformation (Escherichia coli) could be directly used for functional coating on various substrates after simple cell removal from the culture medium without further purification and water-evaporation. This work demonstrates a proof-of-concept of a PET upcycling strategy via a combination of chemo-biological conversion of PET waste into multifunctional coating materials.
Keywords: biocatalysis; catechol; esterase; poly(ethylene terephthalate); upcycling.
© 2021 The Authors. ChemSusChem published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

