Evolutionary Trajectories toward Ceftazidime-Avibactam Resistance in Klebsiella pneumoniae Clinical Isolates
- PMID: 34339281
- PMCID: PMC8448114
- DOI: 10.1128/AAC.00574-21
Evolutionary Trajectories toward Ceftazidime-Avibactam Resistance in Klebsiella pneumoniae Clinical Isolates
Abstract
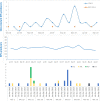
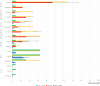
From January 2019 to April 2020, 32 KPC-producing, ceftazidime-avibactam (CZA)-resistant Klebsiella pneumoniae strains were isolated in a university hospital in Rome, Italy. These strains belonged to the sequence type 512 (ST512), ST101, and ST307 high-risk clones. Nine different CZA-resistant KPC-3 protein variants were identified, five of them never previously reported (KPC-66 to KPC-70). Among the nine, KPC-31, KPC-39, KPC-49, KPC-66, KP-68, KPC-69, and KPC-70 showed amino acid substitutions, insertions, and deletions in the Ω loop of the protein. KPC-29 has a duplication, while the novel KPC-67 has a triplication, of the KDD triplet in the 270-loop, a secondary loop of the KPC-3 protein. Genomics performed on contemporary resistant and susceptible clones underlined that these novel mutations emerged in blaKPC-3 genes located on conserved plasmids: in ST512, all blaKPC-3 mutant genes were located in pKpQIL plasmids, while the three novel blaKPC-3 mutants identified in ST101 were on FIIk-FIA(HI1)-R plasmids. Selection also promoted multiplication of the carbapenemase gene copy number by transposition, recombination, and fusion of resident plasmids. When expressed in Escherichia coli recipient cells cloned in the high-copy-number pTOPO vector, the Ω loop mutated variants showed the CZA-resistant phenotype associated with susceptibility to carbapenems, while KPC variants with insertions in the 270-loop showed residual activity on carbapenems. The investigation of CZA resistance mechanisms offered the unique opportunity to study vertical, horizontal, and oblique evolutionary trajectories of K. pneumoniae high-risk clones.
Keywords: Klebsiella pneumoniae; antimicrobial resistance; carbapenemase.
Figures



References
-
- Bassetti M, Giacobbe DR, Giamarellou H, Viscoli C, Daikos GL, Dimopoulos G, De Rosa FG, Giamarellos-Bourboulis EJ, Rossolini GM, Righi E, Karaiskos I, Tumbarello M, Nicolau DP, Viale PL, Poulakou G, Hellenic Society of Chemotherapy (HSC) and Società Italiana di Terapia Antinfettiva (SITA) . 2018. Management of KPC-producing Klebsiella pneumoniae infections. Clin Microbiol Infect 24:133–144. 10.1016/j.cmi.2017.08.030. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

