Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials
- PMID: 34339645
- PMCID: PMC8324484
- DOI: 10.1016/S1470-2045(21)00288-6
Trastuzumab for early-stage, HER2-positive breast cancer: a meta-analysis of 13 864 women in seven randomised trials
Abstract
Background: Trastuzumab targets the extracellular domain of the HER2 protein. Adding trastuzumab to chemotherapy for patients with early-stage, HER2-positive breast cancer reduces the risk of recurrence and death, but is associated with cardiac toxicity. We investigated the long-term benefits and risks of adjuvant trastuzumab on breast cancer recurrence and cause-specific mortality.
Methods: We did a collaborative meta-analysis of individual patient data from randomised trials assessing chemotherapy plus trastuzumab versus the same chemotherapy alone. Randomised trials that enrolled women with node-negative or node-positive, operable breast cancer were included. We collected individual patient-level data on baseline characteristics, dates and sites of first distant breast cancer recurrence and any previous local recurrence or second primary cancer, and the date and underlying cause of death. Primary outcomes were breast cancer recurrence, breast cancer mortality, death without recurrence, and all-cause mortality. Standard intention-to-treat log-rank analyses, stratified by age, nodal status, oestrogen receptor (ER) status, and trial yielded first-event rate ratios (RRs).
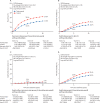
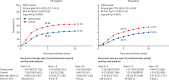
Findings: Seven randomised trials met the inclusion criteria, and included 13 864 patients enrolled between February, 2000, and December, 2005. Mean scheduled treatment duration was 14·4 months and median follow-up was 10·7 years (IQR 9·5 to 11·9). The risks of breast cancer recurrence (RR 0·66, 95% CI 0·62 to 0·71; p<0·0001) and death from breast cancer (0·67, 0·61 to 0·73; p<0·0001) were lower with trastuzumab plus chemotherapy than with chemotherapy alone. Absolute 10-year recurrence risk was reduced by 9·0% (95% CI 7·4 to 10·7; p<0·0001) and 10-year breast cancer mortality was reduced by 6·4% (4·9 to 7·8; p<0·0001), with a 6·5% reduction (5·0 to 8·0; p<0·0001) in all-cause mortality, and no increase in death without recurrence (0·4%, -0·3 to 1·1; p=0·35). The proportional reduction in recurrence was largest in years 0-1 after randomisation (0·53, 99% CI 0·46 to 0·61), with benefits persisting through years 2-4 (0·73, 0·62 to 0·85) and 5-9 (0·80, 0·64 to 1·01), and little follow-up beyond year 10. Proportional recurrence reductions were similar irrespective of recorded patient and tumour characteristics, including ER status. The more high risk the tumour, the larger the absolute reductions in 5-year recurrence (eg, 5·7% [95% CI 3·1 to 8·3], 6·8% [4·7 to 9·0], and 10·7% [7·7 to 13·6] in N0, N1-3, and N4+ disease).
Interpretation: Adding trastuzumab to chemotherapy for early-stage, HER2-positive breast cancer reduces recurrence of, and mortality from, breast cancer by a third, with worthwhile proportional reductions irrespective of recorded patient and tumour characteristics.
Funding: Cancer Research UK, UK Medical Research Council.
Copyright © 2021 The Author(s). Published by Elsevier Ltd. This is an Open Access article under the CC BY 4.0 license. Published by Elsevier Ltd.. All rights reserved.
Figures



References
-
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. - PubMed
-
- Slamon DJ, Leyland-Jones B, Shak S. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344:783–792. - PubMed
-
- Romond EH, Perez EA, Bryant J. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353:1673–1684. - PubMed
-
- Piccart-Gebhart MJ, Procter M, Leyland-Jones B. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353:1659–1672. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

