Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age
- PMID: 34339728
- PMCID: PMC8321962
- DOI: 10.1016/j.jpeds.2021.07.044
Myopericarditis after messenger RNA Coronavirus Disease 2019 Vaccination in Adolescents 12 to 18 Years of Age
Abstract
Objectives: To characterize the clinical course and outcomes of children 12-18 years of age who developed probable myopericarditis after vaccination with the Pfizer-BioNTech (BNT162b2) coronavirus disease 2019 (COVID-19) messenger RNA (mRNA) vaccine.
Study design: A cross-sectional study of 25 children, aged 12-18 years, diagnosed with probable myopericarditis after COVID-19 mRNA vaccination as per the Centers for Disease Control and Prevention criteria for myopericarditis at 8 US centers between May 10, 2021, and June 20, 2021. We retrospectively collected the following data: demographics, severe acute respiratory syndrome coronavirus 2 virus detection or serologic testing, clinical manifestations, laboratory test results, imaging study results, treatment, and time to resolutions of symptoms.
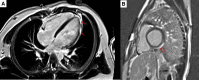
Results: Most (88%) cases followed the second dose of vaccine, and chest pain (100%) was the most common presenting symptom. Patients came to medical attention a median of 2 days (range, <1-20 days) after receipt of Pfizer mRNA COVID-19 vaccination. All adolescents had an elevated plasma troponin concentration. Echocardiographic abnormalities were infrequent, and 92% showed normal cardiac function at presentation. However, cardiac magnetic resonance imaging, obtained in 16 patients (64%), revealed that 15 (94%) had late gadolinium enhancement consistent with myopericarditis. Most were treated with ibuprofen or an equivalent nonsteroidal anti-inflammatory drug for symptomatic relief. One patient was given a corticosteroid orally after the initial administration of ibuprofen or an nonsteroidal anti-inflammatory drug; 2 patients also received intravenous immune globulin. Symptom resolution was observed within 7 days in all patients.
Conclusions: Our data suggest that symptoms owing to myopericarditis after the mRNA COVID-19 vaccination tend to be mild and transient. Approximately two-thirds of patients underwent cardiac magnetic resonance imaging, which revealed evidence of myocardial inflammation despite a lack of echocardiographic abnormalities.
Keywords: mRNA COVID-19 vaccine; myocarditis; pericarditis.
Copyright © 2021. Published by Elsevier Inc.
Figures


Comment in
-
Important Insights into Myopericarditis after the Pfizer mRNA COVID-19 Vaccination in Adolescents.J Pediatr. 2021 Nov;238:5. doi: 10.1016/j.jpeds.2021.07.057. Epub 2021 Jul 29. J Pediatr. 2021. PMID: 34332972 Free PMC article. No abstract available.
References
-
- Wallace M., Woodworth K.R., Gargano J.W., Scobie H.M., Blain A.E., Moulia D., et al. The Advisory Committee on Immunization Practices' interim recommendation for use of Pfizer-BioNTech COVID-19 Vaccine in Adolescents Aged 12-15 Years — United States, May 2021. MMWR Morb Mortal Wkly Rep. 2021;70:749–752. - PMC - PubMed
-
- Centers for Disease Control and Prevention Demographic characterisics of people receiving COVID-19 vaccinations in the United States. https://covid.cdc.gov/covid-data-tracker/#vaccination-demographic
-
- AHA Statement on May 23, 2021. https://newsroom.heart.org/news/covid-19-vaccine-benefits-still-outweigh...
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

