Different roles of T-type calcium channel isoforms in hypnosis induced by an endogenous neurosteroid epipregnanolone
- PMID: 34339750
- PMCID: PMC8478885
- DOI: 10.1016/j.neuropharm.2021.108739
Different roles of T-type calcium channel isoforms in hypnosis induced by an endogenous neurosteroid epipregnanolone
Abstract
Background: Many neuroactive steroids induce sedation/hypnosis by potentiating γ-aminobutyric acid (GABAA) currents. However, we previously demonstrated that an endogenous neuroactive steroid epipregnanolone [(3β,5β)-3-hydroxypregnan-20-one] (EpiP) exerts potent peripheral analgesia and blocks T-type calcium currents while sparing GABAA currents in rat sensory neurons. This study seeks to investigate the behavioral effects elicited by systemic administration of EpiP and to characterize its use as an adjuvant agent to commonly used general anesthetics (GAs).
Methods: Here, we utilized electroencephalographic (EEG) recordings to characterize thalamocortical oscillations, as well as behavioral assessment and mouse genetics with wild-type (WT) and different knockout (KO) models of T-channel isoforms to investigate potential sedative/hypnotic and immobilizing properties of EpiP.
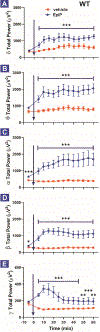
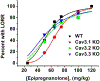
Results: Consistent with increased oscillations in slower EEG frequencies, EpiP induced an hypnotic state in WT mice when injected alone intra-peritoneally (i.p.) and effectively facilitated anesthetic effects of isoflurane (ISO) and sevoflurane (SEVO). The CaV3.1 (Cacna1g) KO mice demonstrated decreased sensitivity to EpiP-induced hypnosis when compared to WT mice, whereas no significant difference was noted between CaV3.2 (Cacna1h), CaV3.3 (Cacna1i) and WT mice. Finally, when compared to WT mice, onset of EpiP-induced hypnosis was delayed in CaV3.2 KO mice but not in CaV3.1 and CaV3.3 KO mice.
Conclusion: We posit that EpiP may have an important role as novel hypnotic and/or adjuvant to volatile anesthetic agents. We speculate that distinct hypnotic effects of EpiP across all three T-channel isoforms is due to their differential expression in thalamocortical circuitry.
Keywords: Calcium; Isoflurane; Low-voltage-activated; Righting reflex; Thalamus; Withdrawal reflex.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Conflict of interest
The authors received no compensation nor do they have any conflicting financial interests in regards to the work described in this manuscript.
Figures




References
-
- Atluri N, Joksimovic SM, Oklopcic A, Milanovic D, Klawitter J, Eggan P, Krishnan K, Covey DF, Todorovic SM, Jevtovic-Todorovic V, 2018. A neurosteroid analogue with T-type calcium channel blocking properties is an effective hypnotic, but is not harmful to neonatal rat brain. Br. J. Anaesth 120, 768–778. - PMC - PubMed
-
- Ayoola C, Hwang SM, Hong SJ, Rose KE, Boyd C, Bozic N, Park JY, Osuru HP, DiGruccio MR, Covey DF, Jevtovic-Todorovic V, Todorovic SM, 2014. Inhibition of CaV3.2 T-type calcium channels in peripheral sensory neurons contributes to analgesic properties of epipregnanolone. Psychopharmacology (Berl). 231, 3503–3515. - PMC - PubMed
-
- Belelli D, Lambert JJ, 2005. Neurosteroids : endogenous regulators of the GABAA receptor. Nat Rev Neurosci 6, 565–575. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

