Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside
- PMID: 34339934
- PMCID: PMC8347670
- DOI: 10.1016/j.ijpddr.2021.07.005
Evaluation of the in vitro susceptibility of various filarial nematodes to emodepside
Abstract
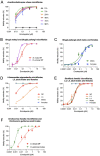
Filariae are vector-borne nematodes responsible for an enormous burden of disease. Human lymphatic filariasis, caused by Wuchereria bancrofti, Brugia malayi, and Brugia timori, and onchocerciasis (caused by Onchocerca volvulus) are neglected parasitic diseases of major public health significance in tropical regions. To date, therapeutic efforts to eliminate human filariasis have been hampered by the lack of a drug with sufficient macrofilaricidal and/or long-term sterilizing effects that is suitable for use in mass drug administration (MDA) programs, particularly in areas co-endemic with Loa loa, the causative agent of loiasis. Emodepside, a semi-synthetic cyclooctadepsipeptide, has been shown to have broad-spectrum efficacy against gastrointestinal nematodes in a variety of mammalian hosts, and has been approved as an active ingredient in dewormers for cats and dogs. This paper evaluates, compares (where appropriate) and summarizes the in vitro effects of emodepside against a range of filarial nematodes at various developmental stages. Emodepside inhibited the motility of all tested stages of filariae frequently used as surrogate species for preclinical investigations (Acanthocheilonema viteae, Brugia pahangi, Litomosoides sigmodontis, Onchocerca gutturosa, and Onchocerca lienalis), human-pathogenic filariae (B. malayi) and filariae of veterinary importance (Dirofilaria immitis) in a concentration-dependent manner. While motility of all filariae was inhibited, both stage- and species-specific differences were observed. However, whether these differences were detected because of stage- and/or species-specific factors or as a consequence of variations in protocol parameters among the participating laboratories (such as purification of the parasites, read-out units, composition of media, incubation conditions, duration of incubation etc.) remains unclear. This study, however, clearly shows that emodepside demonstrates broad-spectrum in vitro activity against filarial nematode species across different genera and can therefore be validated as a promising candidate for the treatment of human filariases, including onchocerciasis and lymphatic filariasis.
Keywords: Anthelmintics; Emodepside; Filariae; Lymphatic filariasis; Onchocerciasis; River blindness.
Copyright © 2021 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Akue J.P. Encephalitis due to Loa loa. In: Tkachev S., editor. Non-flavivirus Encephalitis. InTech; London: 2011.
-
- Bockarie M.J., Deb R.M. Elimination of lymphatic filariasis: do we have the drugs to complete the job? Curr. Opin. Infect. Dis. 2010;23:617–620. - PubMed
-
- Campbell W.C. Vol. 55. Angew. Chem. Int; 2016. Ivermectin: a reflection on simplicity (nobel lecture) pp. 10184–10189. (Engl). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

