The Mammalian Membrane Microenvironment Regulates the Sequential Attachment of Bacteria to Host Cells
- PMID: 34340544
- PMCID: PMC8406306
- DOI: 10.1128/mBio.01392-21
The Mammalian Membrane Microenvironment Regulates the Sequential Attachment of Bacteria to Host Cells
Abstract
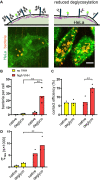
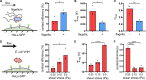
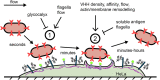
Pathogen attachment to host tissue is critical in the progress of many infections. Bacteria use adhesion in vivo to stabilize colonization and subsequently regulate the deployment of contact-dependent virulence traits. To specifically target host cells, they decorate themselves with adhesins, proteins that bind to mammalian cell surface receptors. One common assumption is that adhesin-receptor interactions entirely govern bacterial attachment. However, how adhesins engage with their receptors in an in vivo-like context remains unclear, in particular under the influence of a heterogeneous mechanical microenvironment. We here investigate the biophysical processes governing bacterial adhesion to host cells using a tunable adhesin-receptor system. By dynamically visualizing attachment, we found that bacterial adhesion to host cell surface, unlike adhesion to inert surfaces, involves two consecutive steps. Bacteria initially attach to their host without engaging adhesins. This step lasts about 1 min, during which bacteria can easily detach. We found that at this stage, the glycocalyx, a layer of glycosylated proteins and lipids, shields the host cell by keeping adhesins away from their receptor ligand. In a second step, adhesins engage with their target receptors to strengthen attachment for minutes to hours. The active properties of the membrane, endowed by the actin cytoskeleton, strengthen specific adhesion. Altogether, our results demonstrate that adhesin-ligand binding is not the sole regulator of bacterial adhesion. In fact, the host cell's surface mechanical microenvironment mediates the physical interactions between host and bacteria, thereby playing an essential role in the onset of infection. IMPORTANCE Microbial adhesion to host cells is the initial step toward many infections. Despite playing a pivotal role in the onset of disease, we still know little about how bacteria attach in an in vivo-like context. By employing a biophysical approach where we investigated host-microbe physical interactions at the single-cell level, we unexpectedly discovered that bacteria attach to mammalian cell membranes in two successive steps. We found that mechanical factors of the cell microenvironment regulate each of these steps, and even dominate biochemical factors, thereby challenging preconceptions on how pathogens interact with their hosts.
Keywords: adhesins; autotransporter proteins; cell adhesion; cell membranes; cytoskeleton; glycocalyx; membrane biophysics; microfluidics.
Figures



Similar articles
-
Bacterial adhesins in host-microbe interactions.Cell Host Microbe. 2009 Jun 18;5(6):580-92. doi: 10.1016/j.chom.2009.05.011. Cell Host Microbe. 2009. PMID: 19527885 Review.
-
Evolution of host-microbe cell adherence by receptor domain shuffling.Elife. 2022 Jan 25;11:e73330. doi: 10.7554/eLife.73330. Elife. 2022. PMID: 35076392 Free PMC article.
-
The cell surface adhesins of Mycobacterium tuberculosis.Microbiol Res. 2020 Feb;232:126392. doi: 10.1016/j.micres.2019.126392. Epub 2019 Dec 9. Microbiol Res. 2020. PMID: 31841935 Review.
-
Burkholderia cenocepacia-host cell contact controls the transcription activity of the trimeric autotransporter adhesin BCAM2418 gene.Microbiologyopen. 2020 Apr;9(4):e998. doi: 10.1002/mbo3.998. Epub 2020 Feb 25. Microbiologyopen. 2020. PMID: 32097539 Free PMC article.
-
Adhesins and receptors of Pseudomonas aeruginosa associated with infection of the respiratory tract.Microb Pathog. 1992 Oct;13(4):251-60. doi: 10.1016/0882-4010(92)90035-m. Microb Pathog. 1992. PMID: 1363702 Review.
Cited by
-
Both LTA and LTB Subunits Are Equally Important to Heat-Labile Enterotoxin (LT)-Enhanced Bacterial Adherence.Int J Mol Sci. 2023 Jan 8;24(2):1245. doi: 10.3390/ijms24021245. Int J Mol Sci. 2023. PMID: 36674760 Free PMC article.
-
Reversible adhesion by type IV pili leads to formation of permanent localized clusters.iScience. 2022 Nov 9;25(12):105532. doi: 10.1016/j.isci.2022.105532. eCollection 2022 Dec 22. iScience. 2022. PMID: 36444306 Free PMC article.
-
Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention.Int J Mol Sci. 2023 Jul 20;24(14):11680. doi: 10.3390/ijms241411680. Int J Mol Sci. 2023. PMID: 37511440 Free PMC article. Review.
-
Effects of a novel Bacillus subtilis GXYX crude lipopeptide against Salmonella enterica serovar Typhimurium infection in mice.Heliyon. 2024 Mar 15;10(6):e28219. doi: 10.1016/j.heliyon.2024.e28219. eCollection 2024 Mar 30. Heliyon. 2024. PMID: 38524560 Free PMC article.
-
A Reversible and Dynamic Surface Functionalization for Fluidity Controlled Multivalent Recognition of Lectins and Bacteria.Adv Sci (Weinh). 2025 Jun;12(22):e2416658. doi: 10.1002/advs.202416658. Epub 2025 Apr 26. Adv Sci (Weinh). 2025. PMID: 40285667 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

