The essential but enigmatic regulatory role of HERVH in pluripotency
- PMID: 34340871
- PMCID: PMC8678302
- DOI: 10.1016/j.tig.2021.07.007
The essential but enigmatic regulatory role of HERVH in pluripotency
Abstract
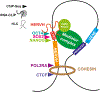
Human specific endogenous retrovirus H (HERVH) is highly expressed in both naive and primed stem cells and is essential for pluripotency. Despite the proven relationship between HERVH expression and pluripotency, there is no single definitive model for the function of HERVH. Instead, several hypotheses of a regulatory function have been put forward including HERVH acting as enhancers, long noncoding RNAs (lncRNAs), and most recently as markers of topologically associating domain (TAD) boundaries. Recently several enhancer-associated lncRNAs have been characterized, which bind to Mediator and are necessary for promoter-enhancer folding interactions. We propose a synergistic model of HERVH function combining relevant findings and discuss the current limitations for its role in regulation, including the lack of evidence for a pluripotency-associated target gene.
Keywords: endogenous retrovirus; enhancer; gene regulation; mediator, topologically associating domain (TAD); stem cell.
Copyright © 2021 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests The authors declare no competing interests.
Figures
References
-
- Lu X et al. (2014) The retrovirus HERVH is a long noncoding RNA required for human embryonic stem cell identity. Nat. Struct. Mol. Biol 21, 423–425 - PubMed
-
- Wang J et al. (2014) Primate-specific endogenous retrovirus-driven transcription defines naive-like stem cells. Nature 516, 405–409 - PubMed
-
- Mager DL and Freeman JD (1995) HERV-H endogenous retroviruses: presence in the new world branch but amplification in the old world primate lineage. Virology 213, 395–404 - PubMed
-
- Jern P et al. (2004) Definition and variation of human endogenous retrovirus H. Virology 327, 93–110 - PubMed
-
- de Parseval N et al. (2001) Characterization of the three HERV-H proviruses with an open envelope reading frame encompassing the immunosuppressive domain and evolutionary history in primates. Virology 279, 558–569 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

