Five-year outcomes of eyes initially enrolled in the 2-year BEVORDEX trial of bevacizumab or dexamethasone implants for diabetic macular oedema
- PMID: 34340975
- PMCID: PMC9763198
- DOI: 10.1136/bjophthalmol-2021-319839
Five-year outcomes of eyes initially enrolled in the 2-year BEVORDEX trial of bevacizumab or dexamethasone implants for diabetic macular oedema
Abstract
Background: The BEVORDEX trial compared outcomes of eyes with diabetic macular oedema (DMO) randomised to receive either intravitreal dexamethasone (DEX-) implant or bevacizumab over 2 years. We assessed long-term efficacy and safety outcomes 5 years from enrolment.
Methods: Patients received standard clinical care after they finished the study. Their files were reviewed for visual and anatomical outcomes, post-trial treatments and complications.
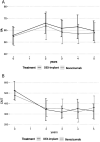
Results: Three-year and five-year data were available for 82% and 59% of eyes enrolled in the BEVORDEX study, respectively. Visual acuity gains at end of trial were generally lost by both treatment groups at 5 years but the macular thickness did not change from end of trial to 5 years. A similar proportion of eyes from each treatment group gained ≥10 letters at 5 years from enrolment in the BEVORDEX trial.Eyes that were initially randomised to the DEX-implant group had significantly fewer treatments but were more likely to develop proliferative diabetic retinopathy (PDR) over the 5-year period compared with eyes initially randomised to bevacizumab. The proportion of eyes that had cataract surgery by 5 years was similar between initial treatment groups.
Conclusions: Eyes in the BEVORDEX trial had similar 5-year rates of cataract surgery, however, more eyes converted to PDR in the group initially treated with DEX-implant. Eyes that were initially treated for 2 years with either intravitreal DEX-implant of bevacizumab followed by standard of care had similar visual and anatomical outcomes at 5 years.
Keywords: macula; treatment medical.
© Author(s) (or their employer(s)) 2023. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: MCG: grant—Allergan; personal fees—Bayer; consultant—Allergan, Novartis, Bayer; expert testimony—Bayer. SF-B: consultant—Allergan, Bayer, Novartis, LLL: consultant—Bayer, Norvartis, Abbvie, Allergan; speaker fees—Bayer, Abbvie. HM: consultant—Allergan, Bayer, Novartis, Roche. KYCT: no financial disclosures. EEC: no financial disclosures. VN: no financial disclosures. ILM: Ad Board—Novartis, Bayer and Allergan. SW: Ad Board—Allergan; speaker fees—Bayer, Allergan.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical