Anticoagulation therapy promotes the tumor immune-microenvironment and potentiates the efficacy of immunotherapy by alleviating hypoxia
- PMID: 34341129
- PMCID: PMC8330593
- DOI: 10.1136/jitc-2021-002332
Anticoagulation therapy promotes the tumor immune-microenvironment and potentiates the efficacy of immunotherapy by alleviating hypoxia
Abstract
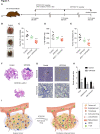
Purpose: Here, this study verifies that cancer-associated thrombosis (CAT) accelerates hypoxia, which is detrimental to the tumor immune microenvironment by limiting tumor perfusion. Therefore, we designed an oral anticoagulant therapy to improve the immunosuppressive tumor microenvironment and potentiate the efficacy of immunotherapy by alleviating tumor hypoxia.
Experimental design: A novel oral anticoagulant (STP3725) was developed to consistently prevent CAT formation. Tumor perfusion and hypoxia were analyzed with or without treating STP3725 in wild-type and P selectin knockout mice. Immunosuppressive cytokines and cells were analyzed to evaluate the alteration of the tumor microenvironment. Effector lymphocyte infiltration in tumor tissue was assessed by congenic CD45.1 mouse lymphocyte transfer model with or without anticoagulant therapy. Finally, various tumor models including K-Ras mutant spontaneous cancer model were employed to validate the role of the anticoagulation therapy in enhancing the efficacy of immunotherapy.
Results: CAT was demonstrated to be one of the perfusion barriers, which fosters immunosuppressive microenvironment by accelerating tumor hypoxia. Consistent treatment of oral anticoagulation therapy was proved to promote tumor immunity by alleviating hypoxia. Furthermore, this resulted in decrease of both hypoxia-related immunosuppressive cytokines and myeloid-derived suppressor cells while improving the spatial distribution of effector lymphocytes and their activity. The anticancer efficacy of αPD-1 antibody was potentiated by co-treatment with STP3725, also confirmed in various tumor models including the K-Ras mutant mouse model, which is highly thrombotic.
Conclusions: Collectively, these findings establish a rationale for a new and translational combination strategy of oral anticoagulation therapy with immunotherapy, especially for treating highly thrombotic cancers. The combination therapy of anticoagulants with immunotherapies can lead to substantial improvements of current approaches in the clinic.
Keywords: adjuvants; immunologic; immunomodulation; tumor microenvironment.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures

Similar articles
-
Salicylic acid-based hypoxia-responsive chemodynamic nanomedicines boost antitumor immunotherapy by modulating immunosuppressive tumor microenvironment.Acta Biomater. 2022 Aug;148:230-243. doi: 10.1016/j.actbio.2022.06.026. Epub 2022 Jun 17. Acta Biomater. 2022. PMID: 35724919
-
Tumor Microenvironment Remodeling by Intratumoral Oncolytic Vaccinia Virus Enhances the Efficacy of Immune-Checkpoint Blockade.Clin Cancer Res. 2019 Mar 1;25(5):1612-1623. doi: 10.1158/1078-0432.CCR-18-1932. Epub 2018 Dec 11. Clin Cancer Res. 2019. PMID: 30538109
-
Alleviating hypoxia to improve cancer immunotherapy.Oncogene. 2023 Dec;42(49):3591-3604. doi: 10.1038/s41388-023-02869-2. Epub 2023 Oct 26. Oncogene. 2023. PMID: 37884747 Review.
-
Targeting hypoxia in the tumor microenvironment: a potential strategy to improve cancer immunotherapy.J Exp Clin Cancer Res. 2021 Jan 9;40(1):24. doi: 10.1186/s13046-020-01820-7. J Exp Clin Cancer Res. 2021. PMID: 33422072 Free PMC article. Review.
-
Blocking Indolamine-2,3-Dioxygenase Rebound Immune Suppression Boosts Antitumor Effects of Radio-Immunotherapy in Murine Models and Spontaneous Canine Malignancies.Clin Cancer Res. 2016 Sep 1;22(17):4328-40. doi: 10.1158/1078-0432.CCR-15-3026. Epub 2016 Mar 15. Clin Cancer Res. 2016. PMID: 26979392 Free PMC article.
Cited by
-
The Plasma DIA-Based Quantitative Proteomics Reveals the Pathogenic Pathways and New Biomarkers in Cervical Cancer and High Grade Squamous Intraepithelial Lesion.J Clin Med. 2022 Dec 1;11(23):7155. doi: 10.3390/jcm11237155. J Clin Med. 2022. PMID: 36498728 Free PMC article.
-
The role of coagulome in the tumor immune microenvironment.Adv Drug Deliv Rev. 2023 Sep;200:115027. doi: 10.1016/j.addr.2023.115027. Epub 2023 Jul 28. Adv Drug Deliv Rev. 2023. PMID: 37517779 Free PMC article. Review.
-
Platelet STING agonism and venous thrombosis: translational implications for improved disease outcomes.J Leukoc Biol. 2023 Sep 1;114(3):195-198. doi: 10.1093/jleuko/qiad073. J Leukoc Biol. 2023. PMID: 37399019 Free PMC article. No abstract available.
-
Therapeutic Strategies Focused on Cancer-Associated Hypercoagulation for Ovarian Clear Cell Carcinoma.Cancers (Basel). 2022 Apr 24;14(9):2125. doi: 10.3390/cancers14092125. Cancers (Basel). 2022. PMID: 35565252 Free PMC article. Review.
-
Venous thrombotic events and impact on outcomes in patients treated with first-line single-agent pembrolizumab in PD-L1 ≥ 50% advanced non small cell lung cancer.J Cancer Res Clin Oncol. 2023 Nov;149(16):15095-15102. doi: 10.1007/s00432-023-05321-w. Epub 2023 Aug 25. J Cancer Res Clin Oncol. 2023. PMID: 37626173 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
