Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children: SIPEXI
- PMID: 34341153
- PMCID: PMC8520391
- DOI: 10.1212/WNL.0000000000012573
Placebo-Controlled Clinical Trial of IncobotulinumtoxinA for Sialorrhea in Children: SIPEXI
Abstract
Background and objectives: To investigate the efficacy and safety of repeated injections of incobotulinumtoxinA (incoBoNT/A) for treatment of chronic sialorrhea (drooling) associated with neurologic disorders (e.g., cerebral palsy, traumatic brain injury) or intellectual disability in children and adolescents in a prospective phase III study (SIPEXI [Sialorrhea Pediatric Xeomin Investigation]).
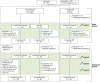
Methods: The study enrolled 2- to 17-year-old patients with sialorrhea due to neurologic disorders or intellectual disability. Patients received body weight-dependent doses of incoBoNT/A (20-75 U). A main period with 1 injection cycle (placebo-controlled, double-blind, 6- to 17-year-olds) was followed by an open-label extension with up to 3 further cycles. An additional cohort of 2- to 5-year-olds received active treatment throughout the study. Coprimary endpoints were the change in unstimulated salivary flow rate (uSFR) from baseline to week 4 and the carers' Global Impression of Change Scale (GICS) rating at week 4. Adverse events were recorded.
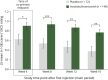
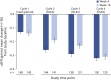
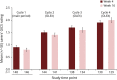
Results: In the main period, 220 patients aged 6-17 years were randomized and treated (148 patients in incoBoNT/A group, 72 patients in placebo group). A total of 35 patients aged 2-5 years received incoBoNT/A (no placebo). A total of 214 patients aged 6-17 years and 33 patients aged 2-5 years continued treatment in the open-label extension period. For the 6- to 17-year-olds, a significant difference between incoBoNT/A and placebo was seen in mean uSFR decrease (difference -0.06 g/min; p = 0.0012) and the carers' GICS rating (difference 0.28 points; p = 0.032) at week 4, in favor of active treatment. The secondary endpoints consistently supported these results. A sustained benefit was observed during the extension. Incidences of adverse events were comparable between incoBoNT/A and placebo and did not increase notably with repeated injections. The most common adverse events were respiratory infections. Efficacy and safety were also favorable in the uncontrolled cohort of 2- to 5-year-olds.
Discussion: Both co-primary efficacy endpoints were reached and superiority of incoBoNT/A over placebo was confirmed. IncoBoNT/A (up to 75 U, up to 4 cycles) is an effective and well-tolerated treatment for sialorrhea associated with neurologic disorders in children.
Trial registration information: Clinicaltrials.gov: NCT02270736 (clinicaltrials.gov/ct2/show/results/NCT02270736); EU Clinical Trials Register: 2013-004532-30 (clinicaltrialsregister.eu/ctr-search/search?query=2013-004532-30).
Classification of evidence: This study provides Class I evidence that injection of incobotulinumtoxinA decreases drooling in children aged 6 to 17 years with neurologic disorders.
Copyright © 2021 The Author(s). Published by Wolters Kluwer Health, Inc. on behalf of the American Academy of Neurology.
Figures
References
-
- Gubbay A, Blackmore MA. Effects of salivary gland botulinum toxin-A on drooling and respiratory morbidity in children with neurological dysfunction. Int J Pediatr Otorhinolaryngol. 2019;124:124-128. - PubMed
-
- Daniel SJ. Multidisciplinary management of sialorrhea in children. Laryngoscope. 2012;122(suppl 4):S67-S68. - PubMed
Associated data
LinkOut - more resources
Full Text Sources
Medical
Research Materials
