Cilastatin Ameliorates Rhabdomyolysis-induced AKI in Mice
- PMID: 34341182
- PMCID: PMC8722809
- DOI: 10.1681/ASN.2020030263
Cilastatin Ameliorates Rhabdomyolysis-induced AKI in Mice
Abstract
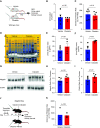
Background: Rhabdomyolysis, the destruction of skeletal muscle, is a significant cause of AKI and death in the context of natural disaster and armed conflict. Rhabdomyolysis may also initiate CKD. Development of specific pharmacologic therapy is desirable because supportive care is nearly impossible in austere environments. Myoglobin, the principal cause of rhabdomyolysis-related AKI, undergoes megalin-mediated endocytosis in proximal tubule cells, a process that specifically injures these cells.
Methods: To investigate whether megalin is protective in a mouse model of rhabdomyolysis-induced AKI, we used male C57BL/6 mice and mice (14-32 weeks old) with proximal tubule-specific deletion of megalin. We used a well-characterized rhabdomyolysis model, injection of 50% glycerol in normal saline preceded by water deprivation.
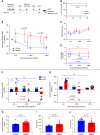
Results: Inducible proximal tubule-specific deletion of megalin was highly protective in this mouse model of rhabdomyolysis-induced AKI. The megalin knockout mice demonstrated preserved GFR, reduced proximal tubule injury (as indicated by kidney injury molecule-1), and reduced renal apoptosis 24 hours after injury. These effects were accompanied by increased urinary myoglobin clearance. Unlike littermate controls, the megalin-deficient mice also did not develop progressive GFR decline and persistent new proteinuria. Administration of the pharmacologic megalin inhibitor cilastatin to wild-type mice recapitulated the renoprotective effects of megalin deletion. This cilastatin-mediated renoprotective effect was dependent on megalin. Cilastatin administration caused selective proteinuria and inhibition of tubular myoglobin uptake similar to that caused by megalin deletion.
Conclusions: We conclude that megalin plays a critical role in rhabdomyolysis-induced AKI, and megalin interference and inhibition ameliorate rhabdomyolysis-induced AKI. Further investigation of megalin inhibition may inform translational investigation of a novel potential therapy.
Keywords: acute renal failure; chronic kidney disease; endocytosis; renal protection; rhabdomyolysis.
Copyright © 2021 by the American Society of Nephrology.
Figures






References
-
- Stewart IJ, Snow BD, Clemens MS, Sosnov JA, Ross JD, Howard JT, et al. : Hyperkalemia in combat casualties: Implications for delayed evacuation. Mil Med 182: e2046–e2051, 2017 - PubMed
-
- Stewart IJ, Faulk TI, Sosnov JA, Clemens MS, Elterman J, Ross JD, et al. : Rhabdomyolysis among critically ill combat casualties: Associations with acute kidney injury and mortality. J Trauma Acute Care Surg 80: 492–498, 2016 - PubMed
-
- Stewart IJ, Sosnov JA, Howard JT, Chung KK: Acute kidney injury in critically injured combat veterans: A retrospective cohort study. Am J Kidney Dis 68: 564–570, 2016 - PubMed
-
- Stewart IJ, Sosnov JA, Howard JT, Orman JA, Fang R, Morrow BD, et al. : Retrospective analysis of long-rerm outcomes after combat injury: A hidden cost of war. Circulation 132: 2126–2133, 2015 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

