Quantifying nanodiamonds biodistribution in whole cells with correlative iono-nanoscopy
- PMID: 34341359
- PMCID: PMC8329174
- DOI: 10.1038/s41467-021-25004-9
Quantifying nanodiamonds biodistribution in whole cells with correlative iono-nanoscopy
Abstract
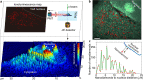
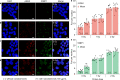
Correlative imaging and quantification of intracellular nanoparticles with the underlying ultrastructure is crucial for understanding cell-nanoparticle interactions in biological research. However, correlative nanoscale imaging of whole cells still remains a daunting challenge. Here, we report a straightforward nanoscopic approach for whole-cell correlative imaging, by simultaneous ionoluminescence and ultrastructure mapping implemented with a highly focused beam of alpha particles. We demonstrate that fluorescent nanodiamonds exhibit fast, ultrabright and stable emission upon excitation by alpha particles. Thus, by using fluorescent nanodiamonds as imaging probes, our approach enables quantification and correlative localization of single nanodiamonds within a whole cell at sub-30 nm resolution. As an application example, we show that our approach, together with Monte Carlo simulations and radiobiological experiments, can be employed to provide unique insights into the mechanisms of nanodiamond radiosensitization at the single whole-cell level. These findings may benefit clinical studies of radio-enhancement effects by nanoparticles in charged-particle cancer therapy.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

