A new silver coordination polymer based on 4,6-diamino-2-pyrimidinethiol: synthesis, characterization and catalytic application in asymmetric Hantzsch synthesis of polyhydroquinolines
- PMID: 34341402
- PMCID: PMC8329208
- DOI: 10.1038/s41598-021-94846-6
A new silver coordination polymer based on 4,6-diamino-2-pyrimidinethiol: synthesis, characterization and catalytic application in asymmetric Hantzsch synthesis of polyhydroquinolines
Abstract
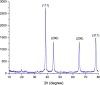
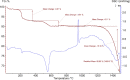
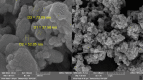
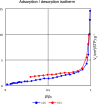
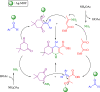
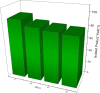
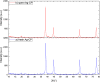
A highly efficient and stable heterogeneous coordination polymer (CP) was successfully prepared by hydrothermal combination of silver and 4,6-diamino-2-pyrimidinethiol. The prepared coordination polymer was characterized by FT-IR, XRD, TGA, SEM, EDX, X-ray mapping and Nitrogen adsorption-desorption analysis. The prepared Ag-CP exhibit excellent catalytic activity in multicomponent Hantzsch synthesis of polyhydroquinolines under mild reaction conditions in relatively short reaction times. The heterogeneity of the catalyst was confirmed by the hot filtration test; also, the catalyst was reused for at least four times under the optimized reaction conditions without any significant loss of its catalytic activity.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures





References
-
- Li M, Wu W, Jiang H. Recent advances in silver-catalyzed transformations of electronically unbiased alkenes and alkynes. ChemCatChem. 2020;12:5034–5050. doi: 10.1002/cctc.202000743. - DOI
-
- Zhang J, et al. Computational advances aiding mechanistic understanding of silver-catalyzed carbene/nitrene/silylene transfer reactions. Coord. Chem. Rev. 2019;382:69–84. doi: 10.1016/j.ccr.2018.12.009. - DOI
-
- Sreedevi R, Saranya S, Anilkumar G. Recent trends in the silver-catalyzed synthesis of nitrogen heterocycles. Adv. Synth. Catal. 2019;361:4625–4644. doi: 10.1002/adsc.201900599. - DOI
-
- Treesa GSS, Saranya S, Meera G, Anilkumar G. Recent advances and perspectives in the silver-catalyzed multi-component reactions. Curr. Org. Chem. 2020;24:291–313. doi: 10.2174/1385272824666200217102036. - DOI
-
- Nathaniel CR, Neetha M, Anilkumar G. Silver-catalyzed pyrrole synthesis: An overview. Appl. Organomet. Chem. 2021 doi: 10.1002/aoc.6141. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

