mTOR signaling mediates ILC3-driven immunopathology
- PMID: 34341503
- PMCID: PMC8528695
- DOI: 10.1038/s41385-021-00432-4
mTOR signaling mediates ILC3-driven immunopathology
Abstract
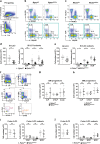
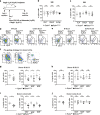
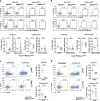
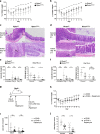
Innate lymphoid cells (ILCs) have a protective immune function at mucosal tissues but can also contribute to immunopathology. Previous work has shown that the serine/threonine kinase mammalian target of rapamycin complex 1 (mTORC1) is involved in generating protective ILC3 cytokine responses during bacterial infection. However, whether mTORC1 also regulates IFN-γ-mediated immunopathology has not been investigated. In addition, the role of mTORC2 in ILC3s is unknown. Using mice specifically defective for either mTORC1 or mTORC2 in ILC3s, we show that both mTOR complexes regulate the maintenance of ILC3s at steady state and pathological immune response during colitis. mTORC1 and to a lesser extend mTORC2 promote the proliferation of ILC3s in the small intestine. Upon activation, intestinal ILC3s produce less IFN-γ in the absence of mTOR signaling. During colitis, loss of both mTOR complexes in colonic ILC3s results in the reduced production of inflammatory mediators, recruitment of neutrophils and immunopathology. Similarly, treatment with rapamycin after colitis induction ameliorates the disease. Collectively, our data show a critical role for both mTOR complexes in controlling ILC3 cell numbers and ILC3-driven inflammation in the intestine.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

