Safe and persistent growth-promoting effects of vosoritide in children with achondroplasia: 2-year results from an open-label, phase 3 extension study
- PMID: 34341520
- PMCID: PMC8327889
- DOI: 10.1038/s41436-021-01287-7
Safe and persistent growth-promoting effects of vosoritide in children with achondroplasia: 2-year results from an open-label, phase 3 extension study
Abstract
Purpose: Achondroplasia is caused by pathogenic variants in the fibroblast growth factor receptor 3 gene that lead to impaired endochondral ossification. Vosoritide, an analog of C-type natriuretic peptide, stimulates endochondral bone growth and is in development for the treatment of achondroplasia. This phase 3 extension study was conducted to document the efficacy and safety of continuous, daily vosoritide treatment in children with achondroplasia, and the two-year results are reported.
Methods: After completing at least six months of a baseline observational growth study, and 52 weeks in a double-blind, placebo-controlled study, participants were eligible to continue treatment in an open-label extension study, where all participants received vosoritide at a dose of 15.0 μg/kg/day.
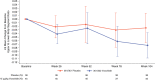
Results: In children randomized to vosoritide, annualized growth velocity increased from 4.26 cm/year at baseline to 5.39 cm/year at 52 weeks and 5.52 cm/year at week 104. In children who crossed over from placebo to vosoritide in the extension study, annualized growth velocity increased from 3.81 cm/year at week 52 to 5.43 cm/year at week 104. No new adverse effects of vosoritide were detected.
Conclusion: Vosoritide treatment has safe and persistent growth-promoting effects in children with achondroplasia treated daily for two years.
© 2021. The Author(s).
Conflict of interest statement
All authors were investigators in this clinical trial except for D.P., K.J., E.F., A.H.L. and J.D., who are employees of the funder (BioMarin). R.S., L.T., F.R. and K.M. have received consulting fees and grants from BioMarin. M.I. and W.W. have received consulting fees from BioMarin. J.C. and D.B. have received grants from BioMarin. LP and PA have received honoraria from BioMarin. C.B. and P.H. have received consulting fees, honoraria and grants from BioMarin. J.H.F. has received consulting fees from BioMarin, Therachon AG and Ascendis, and grants from BioMarin. M.B. has received consulting fees from and grants from BioMarin, Ascendis, Therachon, QED and Alexion; and grants from BioMarin, Ascendis, Therachon, QED, Medlife, SOBI, and Shire. K.W. has received consulting fees from BioMarin and Sanofi/Genzyme, and grants from BioMarin, Ultragenyx, and Ascendis. The other authors declare no competing interests.
Figures