Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function
- PMID: 34341568
- PMCID: PMC7611554
- DOI: 10.1038/s42255-021-00432-5
Leptin brain entry via a tanycytic LepR-EGFR shuttle controls lipid metabolism and pancreas function
Abstract
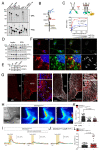
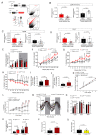
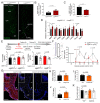
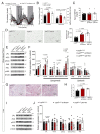
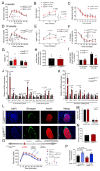
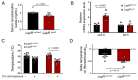
Metabolic health depends on the brain's ability to control food intake and nutrient use versus storage, processes that require peripheral signals such as the adipocyte-derived hormone, leptin, to cross brain barriers and mobilize regulatory circuits. We have previously shown that hypothalamic tanycytes shuttle leptin into the brain to reach target neurons. Here, using multiple complementary models, we show that tanycytes express functional leptin receptor (LepR), respond to leptin by triggering Ca2+ waves and target protein phosphorylation, and that their transcytotic transport of leptin requires the activation of a LepR-EGFR complex by leptin and EGF sequentially. Selective deletion of LepR in tanycytes blocks leptin entry into the brain, inducing not only increased food intake and lipogenesis but also glucose intolerance through attenuated insulin secretion by pancreatic β-cells, possibly via altered sympathetic nervous tone. Tanycytic LepRb-EGFR-mediated transport of leptin could thus be crucial to the pathophysiology of diabetes in addition to obesity, with therapeutic implications.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
The authors have no competing interests to declare.
Figures

References
-
- Swinburn BA, et al. The Global Syndemic of Obesity, Undernutrition, and Climate Change: The Lancet Commission report. Lancet. 2019;393:791–846. - PubMed
-
- Yoon KH, et al. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. - PubMed
-
- Ohn JH, et al. 10-year trajectory of beta-cell function and insulin sensitivity in the development of type 2 diabetes: a community-based prospective cohort study. Lancet Diabetes Endocrinol. 2016;4:27–34. - PubMed
-
- Ahima RS, Flier JS. Leptin. Annu Rev Physiol. 2000;62:413–437. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

