Tumor elastography and its association with cell-free tumor DNA in the plasma of breast tumor patients: a pilot study
- PMID: 34341728
- PMCID: PMC8245960
- DOI: 10.21037/qims-20-443
Tumor elastography and its association with cell-free tumor DNA in the plasma of breast tumor patients: a pilot study
Abstract
Background: Breast tumor stiffness, which can be objectively and noninvasively evaluated by ultrasound elastography (UE), has been useful for the differentiation of benign and malignant breast lesions and the prediction of clinical outcomes. Liquid biopsy analyses, including cell-free tumor DNA (ctDNA), exhibit great potential for personalized treatment. This study aimed to investigate the correlations between the UE and ctDNA for early breast cancer diagnosis.
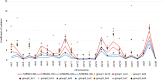
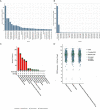
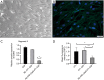
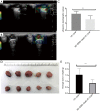
Methods: Breast tumor stiffness in 10 patients were assessed by shear wave elastography (SWE), and the ctDNA of eight collected plasma specimens with different tumor stiffness were analyzed by whole-genome sequencing (WGS). Subsequently, the distribution of carcinoma-associated fibroblasts (CAFs) was investigated by detecting the expression levels of alpha-smooth muscle actin (α-SMA) in tissues of breast lesions. We validated the function of discoidin domain receptor 2 (DDR2) in breast tumor CAFs by knockout of fibroblast activation protein (FAP) with different tumor stiffness during cancer progression in vitro and vivo.
Results: The UE estimates of tumor stiffness positively correlated with CAF-rich (α-SMA+) tumors (P<0.05). Copy number profiles and percent genome alterations were remarkably different between benign and malignant breast lesions. Somatic genomic alterations or structural variants of DDR2, ANTXRL, TPSG1, and TPSB2 genes were identified in ctDNA of plasma from breast lesions with high SWE values and an increase in the CAF content obtained from clinical samples. Deletion of FAP in breast tumor CAFs by CRISPR/Cas9-mediated gene knockout and decreased tumor stiffness resulted in downregulated expression of DDR2 (P<0.05), which in turn led to decreasing the tumor stiffness and carcinogenesis process in vitro and in vivo.
Conclusions: These results have established proof of principle that WGS analysis of ctDNA could complement current UE approaches to assess tumor stiffness changes for the early diagnosis and prognostic assessment of breast cancer.
Keywords: Breast cancer; cell-free tumor DNA (ctDNA); copy number variation (CNV); discoidin domain receptor 2 (DDR2); tumor stiffness.
2021 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-443). All authors reported this study was supported by the general programs from Shenzhen Science and Technology Innovation Committee (JCYJ20190814110207603, JCYJ20190814111801681); the National Natural Science Foundation of China (81972423); the project of free exploration from Shenzhen Science and Technology Innovation Committee (JCYJ20170307144246792, JCYJ20170307144103633); the clinical research start-up plan of Southern Medical University (LC2016YM018); the Incubation Programme of the National Natural Science Foundation of China from Shenzhen Hospital of Southern Medical University (PY2020ZY02); the Social Public Welfare Project of Science and Technology from Shenzhen Baoan District (2016CX301); the Grant of Shenzhen Key Laboratory of Viral Oncology (ZDSYS201707311140430); and the Grant of Shenzhen Sanming Medical Project (SM201702).
Figures




References
LinkOut - more resources
Full Text Sources
Miscellaneous
