Quantitative analysis of renal blood flow during thoracic endovascular aortic repair in type B aortic dissection using syngo iFlow
- PMID: 34341745
- PMCID: PMC8245938
- DOI: 10.21037/qims-20-992
Quantitative analysis of renal blood flow during thoracic endovascular aortic repair in type B aortic dissection using syngo iFlow
Abstract
Background: Currently, the thoracic endovascular aortic repair is the recommended clinical treatment for type B aortic dissections. Unfortunately, malperfusion or ischemia of the kidneys is a major complication of type B aortic dissections. Despite this, few studies have focused on the effects of thoracic endovascular aortic repair on blood flow in renal arteries and parenchyma. This current investigation used novel real-time imaging software to quantitatively analyze the hemodynamic changes in renal artery blood flow and perfusion before and after stent graft placement.
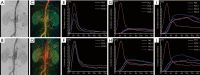
Methods: A total of 51 patients with type B aortic dissection undergoing thoracic endovascular aortic repair between April 2017 and September 2019 were retrospectively recruited. The pre-and post-procedural digital subtraction angiography images were converted into color-coded maps using syngo iFlow for quantitative comparison. Time-intensity curves and related parameters, including the average peak ratio (avg.Pr), average delayed time to peak (avg.dTTP), and average area under the curve ratio (avg.AUCr) of the renal arteries and renal cortex were obtained and analyzed. Wilcoxon signed-rank test was used to compare iFlow parameters before and after endovascular repair. Spearman correlation analyses were performed to study iFlow parameters and renal function parameters and the estimated glomerular filtration rate (eGFR) and blood urea nitrogen (BUN).
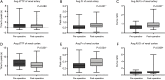
Results: A total of 102 images including 51 pre-operative and 51 post-operative image datasets were successfully post-processed. Following endovascular repair, syngo iFlow showed a significant 33.0% increase in avg.Pr (P<0.001) and a significant 35.1% increase in avg.AUCr (P<0.001) in the renal artery. Additionally, there was a significant 12.2% decrease in the avg.dTTP (P=0.001), a significant 24.5% increase in avg.Pr (P=0.004), and a significant 38.3% increase in avg.AUCr (P=0.009) in the renal cortex. Spearman correlation analysis showed that after endovascular repair there was a significant correlation between the avg.Pr of the renal artery and eGFR (r=0.30; P=0.0349), the avg.Pr of the renal cortex and eGFR (r=0.30; P=0.0300), and the avg. AUCr of the renal cortex and BUN (r=0.31; P=0.0289).
Conclusions: syngo iFlow provided a novel quantitative method for evaluating renal hemodynamic changes in patients with type B aortic dissection undergoing endovascular treatment. Time-intensity curve parameters may facilitate the intraprocedural evaluation of renal blood flow and perfusion to complement the color-coded map.
Keywords: Thoracic endovascular aortic repair (TEVAR); renal blood flow; renal parenchymal perfusion; syngo iFlow; type B aortic dissection (TBAD).
2021 Quantitative Imaging in Medicine and Surgery. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/qims-20-992). The authors have no conflicts of interest to declare.
Figures


Similar articles
-
[Clinical values of hemodynamics assessment by parametric color coding of digital subtraction angiography before and after endovascular therapy for critical limb ischaemia].Zhonghua Yi Xue Za Zhi. 2015 Oct 6;95(37):3036-40. Zhonghua Yi Xue Za Zhi. 2015. PMID: 26814086 Chinese.
-
Clinical validation of 2D perfusion angiography using Syngo iFlow software during peripheral arterial interventions.Vascular. 2021 Jun;29(3):380-386. doi: 10.1177/1708538120957480. Epub 2020 Sep 21. Vascular. 2021. PMID: 32951560
-
Assessment of the re-entry tear index as a prognostic indicator for renal perfusion improvement after thoracic endovascular aortic repair in patients with acute and subacute Stanford type b aortic dissection with renal artery involvement.Quant Imaging Med Surg. 2024 Sep 1;14(9):6222-6237. doi: 10.21037/qims-24-206. Epub 2024 Aug 8. Quant Imaging Med Surg. 2024. PMID: 39281145 Free PMC article.
-
Feasibility study of Syngo iFlow in predicting hemodynamic improvement post-endovascular procedure in peripheral artery disease.BMC Cardiovasc Disord. 2024 Feb 10;24(1):99. doi: 10.1186/s12872-024-03762-w. BMC Cardiovasc Disord. 2024. PMID: 38341562 Free PMC article.
-
Color-coded summation images for the evaluation of blood flow in endovascular aortic dissection fenestration.BMC Med Imaging. 2022 Feb 4;22(1):19. doi: 10.1186/s12880-022-00744-2. BMC Med Imaging. 2022. PMID: 35120493 Free PMC article.
Cited by
-
Angiographic Characteristics of Cerebral Perfusion and Hemodynamics of the Bridging Artery After Surgical Treatment of Unilateral Moyamoya Disease.Front Neurosci. 2022 Jun 14;16:922482. doi: 10.3389/fnins.2022.922482. eCollection 2022. Front Neurosci. 2022. PMID: 35774553 Free PMC article.
-
Effect of prolonged microcirculation time after thrombectomy on the outcome of acute stroke.J Neurointerv Surg. 2023 Nov;15(11):1078-1083. doi: 10.1136/jnis-2022-019566. Epub 2022 Nov 23. J Neurointerv Surg. 2023. PMID: 36418160 Free PMC article.
-
Vesical perfusion volume and internal iliac pressure during double balloon-occluded arterial infusion chemotherapy for bladder cancer.Eur Radiol Exp. 2025 Aug 11;9(1):72. doi: 10.1186/s41747-025-00620-y. Eur Radiol Exp. 2025. PMID: 40790372 Free PMC article.
-
Novel Survival Features Generated by Clinical Text Information and Radiomics Features May Improve the Prediction of Ischemic Stroke Outcome.Diagnostics (Basel). 2022 Jul 8;12(7):1664. doi: 10.3390/diagnostics12071664. Diagnostics (Basel). 2022. PMID: 35885568 Free PMC article.
-
Evaluation of ocular blood flow in the assessment of symptomatic carotid stenosis.Interv Neuroradiol. 2025 Aug;31(4):475-481. doi: 10.1177/15910199231169844. Epub 2023 Apr 17. Interv Neuroradiol. 2025. PMID: 37070150 Free PMC article.
References
-
- Jonker FH, Patel HJ, Upchurch GR, Williams DM, Montgomery DG, Gleason TG, Braverman AC, Sechtem U, Fattori R, Di Eusanio M, Evangelista A, Nienaber CA, Isselbacher EM, Eagle KA, Trimarchi S. Acute type B aortic dissection complicated by visceral ischemia. J Thorac Cardiovasc Surg 2015;149:1081-6.e1. 10.1016/j.jtcvs.2014.11.012 - DOI - PubMed
-
- Shu C, Fang K, Luo M, Li Q, Wang Z. Emergency endovascular stent-grafting for acute type B aortic dissection with symptomatic malperfusion. Int Angiol 2013;32:483-91. - PubMed
-
- Zhang XB, Zhuang ZG, Ye H, Beilner J, Kowarschik M, Chen JJ, Chi JC, Xu JR. Objective assessment of transcatheter arterial chemoembolization angiographic endpoints: preliminary study of quantitative digital subtraction angiography. J Vasc Interv Radiol 2013;24:667-71. 10.1016/j.jvir.2013.01.009 - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
