Abrus precatorius Leaf Extract Reverses Alloxan/Nicotinamide-Induced Diabetes Mellitus in Rats through Hormonal (Insulin, GLP-1, and Glucagon) and Enzymatic (α-Amylase/ α-Glucosidase) Modulation
- PMID: 34341763
- PMCID: PMC8325591
- DOI: 10.1155/2021/9920826
Abrus precatorius Leaf Extract Reverses Alloxan/Nicotinamide-Induced Diabetes Mellitus in Rats through Hormonal (Insulin, GLP-1, and Glucagon) and Enzymatic (α-Amylase/ α-Glucosidase) Modulation
Abstract
Background: Abrus precatorius is used in folk medicine across Afro-Asian regions of the world. Earlier, glucose lowering and pancreato-protective effects of Abrus precatorius leaf extract (APLE) was confirmed experimentally in STZ/nicotinamide-induced diabetic rats; however, the underlying mechanism of antidiabetic effect and pancreato-protection remained unknown.
Objective: This study elucidated antidiabetic mechanisms and pancreato-protective effects of APLE in diabetic rats.
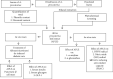
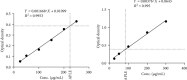
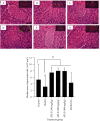
Materials and methods: APLE was prepared by ethanol/Soxhlet extraction method. Total phenols and flavonoids were quantified calorimetrically after initial phytochemical screening. Diabetes mellitus (DM) was established in adult Sprague-Dawley rats (weighing 120-180 g) of both sexes by daily sequential injection of nicotinamide (48 mg/kg; ip) and Alloxan (120 mg/kg; ip) over a period of 7 days. Except control rats which had fasting blood glucose (FBG) of 4.60 mmol/L, rats having stable FBG (16-21 mmol/L) 7 days post-nicotinamide/Alloxan injection were considered diabetic and were randomly reassigned to one of the following groups (model, APLE (100, 200, and 400 mg/kg, respectively; po) and metformin (300 mg/kg; po)) and treated daily for 18 days. Bodyweight and FBG were measured every 72 hours for 18 days. On day 18, rats were sacrificed under deep anesthesia; organs (kidney, liver, pancreas, and spleen) were isolated and weighed. Blood was collected for estimation of serum insulin, glucagon, and GLP-1 using a rat-specific ELISA kit. The pancreas was processed, sectioned, and H&E-stained for histological examination. Effect of APLE on enzymatic activity of alpha (α)-amylase and α-glucosidase was assessed. Antioxidant and free radical scavenging properties of APLE were assessed using standard methods.
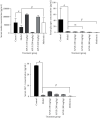
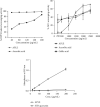
Results: APLE dose-dependently decreased the initial FBG by 68.67%, 31.07%, and 4.39% compared to model (4.34%) and metformin (43.63%). APLE (100 mg/kg) treatment restored weight loss relative to model. APLE increased serum insulin and GLP-1 but decreased serum glucagon relative to model. APLE increased both the number and median crosssectional area (×106 μm2) of pancreatic islets compared to that of model. APLE produced concentration-dependent inhibition of α-amylase and α-glucosidase relative to acarbose. APLE concentration dependently scavenged DPPH and nitric oxide (NO) radicals and demonstrated increased ferric reducing antioxidant capacity (FRAC) relative to standards.
Conclusion: Antidiabetic effect of APLE is mediated through modulation of insulin and GLP-1 inversely with glucagon, noncompetitive inhibition of α-amylase and α-glucosidase, free radical scavenging, and recovery of damaged/necro-apoptosized pancreatic β-cells.
Copyright © 2021 Alex Boye et al.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
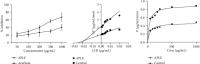

Similar articles
-
Glucose lowering and pancreato-protective effects of Abrus Precatorius (L.) leaf extract in normoglycemic and STZ/Nicotinamide - Induced diabetic rats.J Ethnopharmacol. 2020 Aug 10;258:112918. doi: 10.1016/j.jep.2020.112918. Epub 2020 Apr 30. J Ethnopharmacol. 2020. PMID: 32360561
-
Antioxidant, α-glucosidase inhibitory activity and sub-chronic toxicity of Derris reticulata extract: its antidiabetic potential.BMC Complement Altern Med. 2015 Feb 27;15:35. doi: 10.1186/s12906-015-0552-4. BMC Complement Altern Med. 2015. PMID: 25887793 Free PMC article.
-
Antidiabetic and antioxidant potential of Alnus nitida leaves in alloxan induced diabetic rats.J Ethnopharmacol. 2020 Apr 6;251:112544. doi: 10.1016/j.jep.2020.112544. Epub 2020 Jan 3. J Ethnopharmacol. 2020. PMID: 31904496
-
Molecular Mechanisms Underlying the Therapeutic Potential of Plant-Based α-Amylase Inhibitors for Hyperglycemic Control in Diabetes.Curr Diabetes Rev. 2025;21(8):e020724231486. doi: 10.2174/0115733998304373240611110224. Curr Diabetes Rev. 2025. PMID: 38956911 Review.
-
Insights into the cellular, molecular, and epigenetic targets of gamma-aminobutyric acid against diabetes: a comprehensive review on its mechanisms.Crit Rev Food Sci Nutr. 2024;64(33):12620-12637. doi: 10.1080/10408398.2023.2255666. Epub 2023 Sep 11. Crit Rev Food Sci Nutr. 2024. PMID: 37694998 Review.
Cited by
-
4-Methoxylonchocarpin protects against cisplatin induced acute kidney injury via regulating ferroptosis.Ren Fail. 2025 Dec;47(1):2545941. doi: 10.1080/0886022X.2025.2545941. Epub 2025 Aug 12. Ren Fail. 2025. PMID: 40796806 Free PMC article.
-
Neurotoxic effects of type II-diabetes mellitus and the possible preventive effects of olive leaves supplement in male rats.Open Vet J. 2024 Oct;14(10):2651-2661. doi: 10.5455/OVJ.2024.v14.i10.15. Epub 2024 Oct 31. Open Vet J. 2024. PMID: 39545190 Free PMC article.
-
N-Acetyl Cysteine, Selenium, and Ascorbic Acid Rescue Diabetic Cardiac Hypertrophy via Mitochondrial-Associated Redox Regulators.Molecules. 2021 Nov 30;26(23):7285. doi: 10.3390/molecules26237285. Molecules. 2021. PMID: 34885867 Free PMC article.
-
Pharmacologically Active Phytomolecules Isolated from Traditional Antidiabetic Plants and Their Therapeutic Role for the Management of Diabetes Mellitus.Molecules. 2022 Jul 3;27(13):4278. doi: 10.3390/molecules27134278. Molecules. 2022. PMID: 35807526 Free PMC article. Review.
-
A Single Strain of Lactobacillus (CGMCC 21661) Exhibits Stable Glucose- and Lipid-Lowering Effects by Regulating Gut Microbiota.Nutrients. 2023 Jan 28;15(3):670. doi: 10.3390/nu15030670. Nutrients. 2023. PMID: 36771383 Free PMC article.
References
-
- Boye A., Acheampong D. O., Gyamerah E. O., et al. Glucose lowering and pancreato-protective effects of Abrus Precatorius (L.) leaf extract in normoglycemic and STZ/nicotinamide-induced diabetic rats. Journal of ethnopharmacology. 2020;258, article 112918 - PubMed
-
- Bettencourt-Silva R., Aguiar B., Sá-Araújo V., et al. Diabetes-related symptoms, acute complications and management of diabetes mellitus of patients who are receiving palliative care: a protocol for a systematic review. BMJ open. 2019;9(6, article e028604) doi: 10.1136/bmjopen-2018-028604. - DOI - PMC - PubMed
-
- Rehman K., Akash M. S. H. Mechanism of generation of oxidative stress and pathophysiology of type 2 diabetes mellitus: how are they interlinked? Journal of cellular biochemistry. 2017;118(11):3577–3585. - PubMed
-
- Archvadze A., Kistauri A., Gongadze N., Makharadze T., Chirakadze K. Medical basis of diabetic neuropathy formation (review) Georgian Medical News. 2018;283:154–162. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

