CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2
- PMID: 34341769
- PMCID: PMC8265543
- DOI: 10.1021/acscentsci.0c01537
CD209L/L-SIGN and CD209/DC-SIGN Act as Receptors for SARS-CoV-2
Abstract
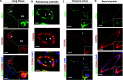
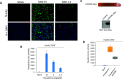
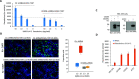
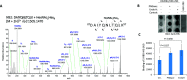
As the COVID-19 pandemic continues to spread, investigating the processes underlying the interactions between SARS-CoV-2 and its hosts is of high importance. Here, we report the identification of CD209L/L-SIGN and the related protein CD209/DC-SIGN as receptors capable of mediating SARS-CoV-2 entry into human cells. Immunofluorescence staining of human tissues revealed prominent expression of CD209L in the lung and kidney epithelia and endothelia. Multiple biochemical assays using a purified recombinant SARS-CoV-2 spike receptor-binding domain (S-RBD) or S1 encompassing both N termal domain and RBD and ectopically expressed CD209L and CD209 revealed that CD209L and CD209 interact with S-RBD. CD209L contains two N-glycosylation sequons, at sites N92 and N361, but we determined that only site N92 is occupied. Removal of the N-glycosylation at this site enhances the binding of S-RBD with CD209L. CD209L also interacts with ACE2, suggesting a role for heterodimerization of CD209L and ACE2 in SARS-CoV-2 entry and infection in cell types where both are present. Furthermore, we demonstrate that human endothelial cells are permissive to SARS-CoV-2 infection, and interference with CD209L activity by a knockdown strategy or with soluble CD209L inhibits virus entry. Our observations demonstrate that CD209L and CD209 serve as alternative receptors for SARS-CoV-2 in disease-relevant cell types, including the vascular system. This property is particularly important in tissues where ACE2 has low expression or is absent and may have implications for antiviral drug development.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

Update of
-
CD209L/L-SIGN and CD209/DC-SIGN act as receptors for SARS-CoV-2.bioRxiv [Preprint]. 2021 Jun 14:2020.06.22.165803. doi: 10.1101/2020.06.22.165803. bioRxiv. 2021. Update in: ACS Cent Sci. 2021 Jul 28;7(7):1156-1165. doi: 10.1021/acscentsci.0c01537. PMID: 32607506 Free PMC article. Updated. Preprint.
References
-
- Bhatraju P. K.; Ghassemieh B. J.; Nichols M.; Kim R.; Jerome K. R.; Nalla A. K.; Greninger A. L.; Pipavath S.; Wurfel M. M.; Evans L.; Kritek P. A.; West T. E.; Luks A.; Gerbino A.; Dale C. R.; Goldman J. D.; O’Mahony S.; Mikacenic C. Covid-19 in Critically Ill Patients in the Seattle Region-Case Series. N. Engl. J. Med. 2020, 382, 2012.10.1056/NEJMoa2004500. - DOI - PMC - PubMed
-
- Huang C.; Wang Y.; Li X.; Ren L.; Zhao J.; Hu Y.; Zhang L.; Fan G.; Xu J.; Gu X.; Cheng Z.; Yu T.; Xia J.; Wei Y.; Wu W.; Xie X.; Yin W.; Li H.; Liu M.; Xiao Y.; Gao H.; Guo L.; Xie J.; Wang G.; Jiang R.; Gao Z.; Jin Q.; Wang J.; Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395 (10223), 497–506. 10.1016/S0140-6736(20)30183-5. - DOI - PMC - PubMed
Grants and funding
- S10 OD021728/OD/NIH HHS/United States
- UC7 AI095321/AI/NIAID NIH HHS/United States
- R21 CA193958/CA/NCI NIH HHS/United States
- T32 HL007035/HL/NHLBI NIH HHS/United States
- T32 GM007753/GM/NIGMS NIH HHS/United States
- R24 GM134210/GM/NIGMS NIH HHS/United States
- R01 AI146779/AI/NIAID NIH HHS/United States
- T32 GM008313/GM/NIGMS NIH HHS/United States
- R01 AI064099/AI/NIAID NIH HHS/United States
- R01 HL132325/HL/NHLBI NIH HHS/United States
- UL1 TR001430/TR/NCATS NIH HHS/United States
- T32 AI007245/AI/NIAID NIH HHS/United States
- R01 AG060890/AG/NIA NIH HHS/United States
- S10 OD010724/OD/NIH HHS/United States
- R21 CA191970/CA/NCI NIH HHS/United States
- R01 AI133486/AI/NIAID NIH HHS/United States
- P30 AI042853/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

