Janus Emulsion Biosensors for Anti-SARS-CoV-2 Spike Antibody
- PMID: 34341770
- PMCID: PMC8231659
- DOI: 10.1021/acscentsci.1c00173
Janus Emulsion Biosensors for Anti-SARS-CoV-2 Spike Antibody
Abstract
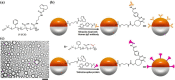
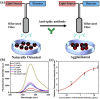
The spread of the COVID-19 pandemic around the world has revealed that it is urgently important to develop rapid and inexpensive assays for antibodies in general and anti-SARS-CoV-2 IgG antibody (anti-SARS-CoV-2 spike glycoprotein S1 antibody) in particular. Herein we report a method to detect the anti-SARS-CoV-2 spike antibody level by using Janus emulsions or Janus particles as biosensors. Janus emulsions are composed of two immiscible hydrocarbon and fluorocarbon oils. The hydrocarbon/water interfaces are functionalized with a secondary antibody of IgG protein and SARS-CoV-2 spike receptor binding domain (RBD), to produce two different Janus emulsions. Mixtures of these Janus droplets enable the detection of the anti-SARS-CoV-2 spike IgG antibody in an agglutination assay caused by the antibody's binding to both the secondary antibody of IgG antibody and SARS-CoV-2 spike protein RBD. Both qualitative optical images and quantitative fluorescence spectra are able to detect the level of anti-SARS-CoV-2 spike antibody at concentrations as low as 0.2 μg/mL in 2 h. The detection results of clinical human serum samples using this agglutination assay confirm that this method is applicable to clinical samples with good sensitivity and specificity. The reported method is generalizable and can be used to detect other analytes by attaching different biomolecular recognition elements to the surface of the Janus droplets.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





References
LinkOut - more resources
Full Text Sources
Miscellaneous

