Homogeneous Gold Redox Chemistry: Organometallics, Catalysis, and Beyond
- PMID: 34341775
- PMCID: PMC8321390
- DOI: 10.1016/j.trechm.2020.04.012
Homogeneous Gold Redox Chemistry: Organometallics, Catalysis, and Beyond
Abstract
Gold redox chemistry holds the promise of unique reactivities and selectivities that are different to other transition metals. Recent studies have utilized strain release, ligand design, and photochemistry to promote the otherwise sluggish oxidative addition to Au(I) complexes. More details on the reductive elimination from Au(III) complexes have also been revealed. These discoveries have facilitated the development of gold redox catalysis and will continue to offer mechanistic insight and inspiration for other transition metals. This review highlights how research in organometallic chemistry has led to gold redox catalysis, as well as applications in materials science, bioconjugation, and radiochemical synthesis.
Figures

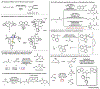



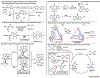
References
-
- Hashmi ASK (2007) Gold-catalyzed organic reactions. Chem. Rev. 107, 3180–3211 - PubMed
-
- Gorin DJ and Toste FD (2007) Relativistic effects in homogeneous gold catalysis. Nature 446, 395–403 - PubMed
-
- Fürstner A and Davies PW (2007) Catalytic carbophilic activation: catalysis by platinum and gold π acids. Angew. Chem. Int. Ed. 46, 3410–3449 - PubMed
-
- He C et al. (2008) Gold-catalyzed organic transformations. Chem. Rev. 108, 3239–3265 - PubMed
