Modified UCN2 peptide treatment improves skeletal muscle mass and function in mouse models of obesity-induced insulin resistance
- PMID: 34342159
- PMCID: PMC8517345
- DOI: 10.1002/jcsm.12746
Modified UCN2 peptide treatment improves skeletal muscle mass and function in mouse models of obesity-induced insulin resistance
Abstract
Background: Type 2 diabetes and obesity are often seen concurrently with skeletal muscle wasting, leading to further derangements in function and metabolism. Muscle wasting remains an unmet need for metabolic disease, and new approaches are warranted. The neuropeptide urocortin 2 (UCN2) and its receptor corticotropin releasing factor receptor 2 (CRHR2) are highly expressed in skeletal muscle and play a role in regulating energy balance, glucose metabolism, and muscle mass. The aim of this study was to investigate the effects of modified UCN2 peptides as a pharmaceutical therapy to counteract the loss of skeletal muscle mass associated with obesity and casting immobilization.
Methods: High-fat-fed mice (C57Bl/6J; 26 weeks old) and ob/ob mice (11 weeks old) were injected daily with a PEGylated (Compound A) and non-PEGylated (Compound B) modified human UCN2 at 0.3 mg/kg subcutaneously for 14 days. A separate group of chow-fed C57Bl/6J mice (12 weeks old) was subjected to hindlimb cast immobilization and, after 1 week, received daily injections with Compound A. In vivo functional tests were performed to measure protein synthesis rates and skeletal muscle function. Ex vivo functional and molecular tests were performed to measure contractile force and signal transduction of catabolic and anabolic pathways in skeletal muscle.
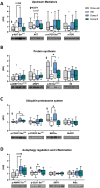
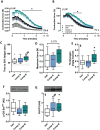
Results: Skeletal muscles (extensor digitorum longus, soleus, and tibialis anterior) from high-fat-fed mice treated with Compound A were ~14% heavier than muscles from vehicle-treated mice. Chronic treatment with modified UCN2 peptides altered the expression of structural genes and transcription factors in skeletal muscle in high-fat diet-induced obesity including down-regulation of Trim63 and up-regulation of Nr4a2 and Igf1 (P < 0.05 vs. vehicle). Signal transduction via both catabolic and anabolic pathways was increased in tibialis anterior muscle, with increased phosphorylation of ribosomal protein S6 at Ser235/236 , FOXO1 at Ser256 , and ULK1 at Ser317 , suggesting that UCN2 treatment modulates protein synthesis and degradation pathways (P < 0.05 vs. vehicle). Acutely, a single injection of Compound A in drug-naïve mice had no effect on the rate of protein synthesis in skeletal muscle, as measured via the surface sensing of translation method, while the expression of Nr4a3 and Ppargc1a4 was increased (P < 0.05 vs. vehicle). Compound A treatment prevented the loss of force production from disuse due to casting. Compound B treatment increased time to fatigue during ex vivo contractions of fast-twitch extensor digitorum longus muscle. Compound A and B treatment increased lean mass and rates of skeletal muscle protein synthesis in ob/ob mice.
Conclusions: Modified human UCN2 is a pharmacological candidate for the prevention of the loss of skeletal muscle mass associated with obesity and immobilization.
Keywords: Diabetes; Exercise; Insulin resistance; Muscle wasting; Obesity; Therapy.
© 2021 The Authors. Journal of Cachexia, Sarcopenia and Muscle published by John Wiley & Sons Ltd on behalf of Society on Sarcopenia, Cachexia and Wasting Disorders.
Conflict of interest statement
J.A.F., B.Y., A.C., and J.T.B. are employees of Eli Lilly and Company. J.R.Z. received Compounds A and B as a gift from Eli Lilly and Company. No other potential conflicts of interest relevant to this article were reported.
Figures





References
-
- Organization WH . Geneva. Global report on diabetes http://wwwwhoint/diabetes/global‐report/en/ 2006.
-
- Narici MV, Maffulli N. Sarcopenia: characteristics, mechanisms and functional significance. Br Med Bull 2010;95:139–159. - PubMed
-
- Vandervoort AA. Aging of the human neuromuscular system. Muscle Nerve 2002;25:17–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

