Management Approach to Acute Myeloid Leukemia Leveraging the Available Resources in View of the Latest Evidence: Consensus of the Saudi Society of Blood and Marrow Transplantation
- PMID: 34343012
- PMCID: PMC8457782
- DOI: 10.1200/GO.20.00660
Management Approach to Acute Myeloid Leukemia Leveraging the Available Resources in View of the Latest Evidence: Consensus of the Saudi Society of Blood and Marrow Transplantation
Abstract
Purpose: Acute myeloid leukemia (AML) is the most prevalent acute leukemia in adults and is responsible for the majority of cancer-related mortality. In Saudi Arabia, leukemia is ranked the fifth most prevalent type of malignancy in adults. Our aim is to review existing epidemiologic data in Saudi Arabia and develop consensus guidelines for management of AML.
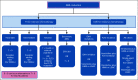
Methods: We review literature related to AML epidemiology, treatment patterns, and outcomes in Saudi Arabia, as well as literature related to the current advances in AML treatment. A panel of 10 experts from eight institutions in Saudi Arabia reviewed the literature and developed a consensus statement.
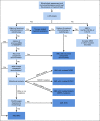
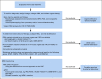
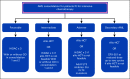
Result: We provide an update of the available AML epidemiologic data in Saudi Arabia and describe recent developments in the diagnostic workup, risk stratification, and treatment algorithm. The consensus recommendations for the management of AML in Saudi Arabia were developed.
Conclusion: The recommendations are in parallel with the recent international guidelines for the diagnosis and management of AML.
Conflict of interest statement
Figures

References
-
- Meyers J Yu Y Kaye JA, et al. : Medicare fee-for-service enrollees with primary acute myeloid leukemia: An analysis of treatment patterns, survival, and healthcare resource utilization and costs. Appl Health Econ Health Policy 11:275-286, 2013 - PubMed
-
- Miranda-Filho A Piñeros M Ferlay J, et al. : Epidemiological patterns of leukaemia in 184 countries: A population-based study. Lancet Haematol 5:e14-e24, 2018 - PubMed
-
- Cancer stat facts: Leukemia—acute myeloid leukemia (AML). https://seer.cancer.gov/statfacts/html/amyl.html
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

