Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study
- PMID: 34343033
- PMCID: PMC8478403
- DOI: 10.1200/JCO.21.00217
Avapritinib Versus Regorafenib in Locally Advanced Unresectable or Metastatic GI Stromal Tumor: A Randomized, Open-Label Phase III Study
Abstract
Purpose: Primary or secondary mutations in KIT or platelet-derived growth factor receptor alpha (PDGFRA) underlie tyrosine kinase inhibitor resistance in most GI stromal tumors (GISTs). Avapritinib selectively and potently inhibits KIT- and PDGFRA-mutant kinases. In the phase I NAVIGATOR study (NCT02508532), avapritinib showed clinical activity against PDGFRA D842V-mutant and later-line KIT-mutant GIST. VOYAGER (NCT03465722), a phase III study, evaluated efficacy and safety of avapritinib versus regorafenib as third-line or later treatment in patients with unresectable or metastatic GIST.
Patients and methods: VOYAGER randomly assigned patients 1:1 to avapritinib 300 mg once daily (4 weeks continuously) or regorafenib 160 mg once daily (3 weeks on and 1 week off). Primary end point was progression-free survival (PFS) by central radiology per RECIST version 1.1 modified for GIST. Secondary end points included objective response rate, overall survival, safety, disease control rate, and duration of response. Regorafenib to avapritinib crossover was permitted upon centrally confirmed disease progression.
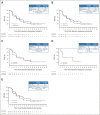
Results: Four hundred seventy-six patients were randomly assigned (avapritinib, n = 240; regorafenib, n = 236). Median PFS was not statistically different between avapritinib and regorafenib (hazard ratio, 1.25; 95% CI, 0.99 to 1.57; 4.2 v 5.6 months; P = .055). Overall survival data were immature at cutoff. Objective response rates were 17.1% and 7.2%, with durations of responses of 7.6 and 9.4 months for avapritinib and regorafenib; disease control rates were 41.7% (95% CI, 35.4 to 48.2) and 46.2% (95% CI, 39.7 to 52.8). Treatment-related adverse events (any grade, grade ≥ 3) were similar for avapritinib (92.5% and 55.2%) and regorafenib (96.2% and 57.7%).
Conclusion: Primary end point was not met. There was no significant difference in median PFS between avapritinib and regorafenib in patients with molecularly unselected, late-line GIST.
Conflict of interest statement
Figures
References
-
- Nilsson B, Bumming P, Meis-Kindblom JM, et al. : Gastrointestinal stromal tumors: The incidence, prevalence, clinical course, and prognostication in the preimatinib mesylate era--a population-based study in western Sweden. Cancer 103:821-829, 2005 - PubMed
-
- Soreide K, Sandvik OM, Soreide JA, et al. : Global epidemiology of gastrointestinal stromal tumours (GIST): A systematic review of population-based cohort studies. Cancer Epidemiol 40:39-46, 2016 - PubMed
-
- Casali PG, Abecassis N, Aro HT, et al. : Gastrointestinal stromal tumours: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 29:iv68-iv78, 2018 - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

