OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice
- PMID: 34343171
- PMCID: PMC8363014
- DOI: 10.1371/journal.pgen.1009699
OsMADS23 phosphorylated by SAPK9 confers drought and salt tolerance by regulating ABA biosynthesis in rice
Abstract
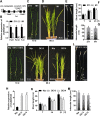
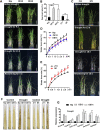
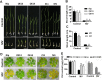
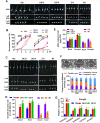
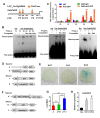
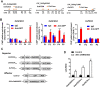
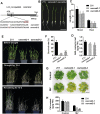
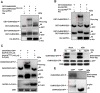
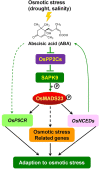
Some of MADS-box transcription factors (TFs) have been shown to play essential roles in the adaptation of plant to abiotic stress. Still, the mechanisms that MADS-box proteins regulate plant stress response are not fully understood. Here, a stress-responsive MADS-box TF OsMADS23 from rice conferring the osmotic stress tolerance in plants is reported. Overexpression of OsMADS23 remarkably enhanced, but knockout of the gene greatly reduced the drought and salt tolerance in rice plants. Further, OsMADS23 was shown to promote the biosynthesis of endogenous ABA and proline by activating the transcription of target genes OsNCED2, OsNCED3, OsNCED4 and OsP5CR that are key components for ABA and proline biosynthesis, respectively. Then, the convincing evidence showed that the OsNCED2-knockout mutants had lower ABA levels and exhibited higher sensitivity to drought and oxidative stress than wild type, which is similar to osmads23 mutant. Interestingly, the SnRK2-type protein kinase SAPK9 was found to physically interact with and phosphorylate OsMADS23, and thus increase its stability and transcriptional activity. Furthermore, the activation of OsMADS23 by SAPK9-mediated phosphorylation is dependent on ABA in plants. Collectively, these findings establish a mechanism that OsMADS23 functions as a positive regulator in response to osmotic stress by regulating ABA biosynthesis, and provide a new strategy for improving drought and salt tolerance in rice.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
The sucrose non-fermenting 1-related kinase 2 gene SAPK9 improves drought tolerance and grain yield in rice by modulating cellular osmotic potential, stomatal closure and stress-responsive gene expression.BMC Plant Biol. 2016 Jul 13;16(1):158. doi: 10.1186/s12870-016-0845-x. BMC Plant Biol. 2016. PMID: 27411911 Free PMC article.
-
Rice OsPUB16 modulates the 'SAPK9-OsMADS23-OsAOC' pathway to reduce plant water-deficit tolerance by repressing ABA and JA biosynthesis.PLoS Genet. 2022 Nov 28;18(11):e1010520. doi: 10.1371/journal.pgen.1010520. eCollection 2022 Nov. PLoS Genet. 2022. PMID: 36441771 Free PMC article.
-
Overexpression of OsMYB48-1, a novel MYB-related transcription factor, enhances drought and salinity tolerance in rice.PLoS One. 2014 Mar 25;9(3):e92913. doi: 10.1371/journal.pone.0092913. eCollection 2014. PLoS One. 2014. PMID: 24667379 Free PMC article.
-
Pivotal role of the AREB/ABF-SnRK2 pathway in ABRE-mediated transcription in response to osmotic stress in plants.Physiol Plant. 2013 Jan;147(1):15-27. doi: 10.1111/j.1399-3054.2012.01635.x. Epub 2012 May 16. Physiol Plant. 2013. PMID: 22519646 Review.
-
Cross-talk between abscisic acid-dependent and abscisic acid-independent pathways during abiotic stress.Plant Cell Rep. 2013 Jul;32(7):985-1006. doi: 10.1007/s00299-013-1414-5. Epub 2013 Mar 19. Plant Cell Rep. 2013. PMID: 23508256 Review.
Cited by
-
Molecular Mechanisms and Regulatory Pathways Underlying Drought Stress Response in Rice.Int J Mol Sci. 2024 Jan 18;25(2):1185. doi: 10.3390/ijms25021185. Int J Mol Sci. 2024. PMID: 38256261 Free PMC article. Review.
-
OsNAC120 balances plant growth and drought tolerance by integrating GA and ABA signaling in rice.Plant Commun. 2024 Mar 11;5(3):100782. doi: 10.1016/j.xplc.2023.100782. Epub 2023 Dec 26. Plant Commun. 2024. PMID: 38148603 Free PMC article.
-
OsPP65 Negatively Regulates Osmotic and Salt Stress Responses Through Regulating Phytohormone and Raffinose Family Oligosaccharide Metabolic Pathways in Rice.Rice (N Y). 2022 Jul 2;15(1):34. doi: 10.1186/s12284-022-00581-5. Rice (N Y). 2022. PMID: 35779169 Free PMC article.
-
The AGAMOUS-LIKE 16-GENERAL REGULATORY FACTOR 1 module regulates axillary bud outgrowth via catabolism of abscisic acid in cucumber.Plant Cell. 2024 Jul 2;36(7):2689-2708. doi: 10.1093/plcell/koae108. Plant Cell. 2024. PMID: 38581430 Free PMC article.
-
Genetic Dissection of Salt Tolerance and Yield Traits of Geng (japonica) Rice by Selective Subspecific Introgression.Curr Issues Mol Biol. 2023 May 31;45(6):4796-4813. doi: 10.3390/cimb45060305. Curr Issues Mol Biol. 2023. PMID: 37367054 Free PMC article.
References
-
- Nakashima K, Tran LSP, Van Nguyen D, Fujita M, Maruyama K, Todaka D, et al.. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007;51(4):617–30. doi: 10.1111/j.1365-313X.2007.03168.x WOS:000249203100007. - DOI - PubMed
-
- Yan H, Jia H, Chen X, Hao L, An H, Guo X. The cotton WRKY transcription factor GhWRKY17 functions in drought and salt stress in transgenic Nicotiana benthamiana through ABA signaling and the modulation of reactive oxygen species production. Plant Cell Physiol. 2014;55(12):2060–76. doi: 10.1093/pcp/pcu133 WOS:000348612000004. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

