Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 74,754 patients
- PMID: 34343191
- PMCID: PMC8330918
- DOI: 10.1371/journal.pone.0255541
Examining the potential benefits of the influenza vaccine against SARS-CoV-2: A retrospective cohort analysis of 74,754 patients
Abstract
Introduction: Recently, several single center studies have suggested a protective effect of the influenza vaccine against severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2). This study utilizes a continuously updated Electronic Medical Record (EMR) network to assess the possible benefits of influenza vaccination mitigating critical adverse outcomes in SARS-CoV-2 positive patients from 56 healthcare organizations (HCOs).
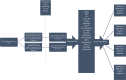
Methods: The de-identified records of 73,346,583 patients were retrospectively screened. Two cohorts of 37,377 patients, having either received or not received influenza vaccination six months-two weeks prior to SARS-CoV-2 positive diagnosis, were created using Common Procedural Terminology (CPT) and logical observation identifiers names and codes (LOINC) codes. Adverse outcomes within 30, 60, 90, and 120 days of positive SARS-CoV-2 diagnosis were compared between cohorts. Outcomes were assessed with stringent propensity score matching including age, race, ethnicity, gender, hypertension, diabetes, hyperlipidemia, chronic obstructive pulmonary disease (COPD), obesity, heart disease, and lifestyle habits such as smoking.
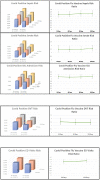
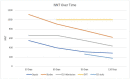
Results: SARS-CoV-2-positive patients who received the influenza vaccine experienced decreased sepsis (p<0.01, Risk Ratio: 1.361-1.450, 95% CI:1.123-1.699, NNT:286) and stroke (p<0.02, RR: 1.451-1.580, 95% CI:1.075-2.034, NNT:625) across all time points. ICU admissions were lower in SARS-CoV-2-positive patients receiving the influenza vaccine at 30, 90, and 120 days (p<0.03, RR: 1.174-1.200, 95% CI:1.003-1.385, NNT:435), while approaching significance at 60 days (p = 0.0509, RR: 1.156, 95% CI:0.999-1.338). Patients who received the influenza vaccine experienced fewer DVTs 60-120 days after positive SARS-CoV-2 diagnosis (p<0.02, RR:1.41-1.530, 95% CI:1.082-2.076, NNT:1000) and experienced fewer emergency department (ED) visits 90-120 days post SARS-CoV-2-positive diagnosis (p<0.01, RR:1.204-1.580, 95% CI: 1.050-1.476, NNT:176).
Conclusion: Our analysis outlines the potential protective effect of influenza vaccination in SARS-CoV-2-positive patients against adverse outcomes within 30, 60, 90, and 120 days of a positive diagnosis. Significant findings favoring influenza vaccination mitigating the risks of sepsis, stroke, deep vein thrombosis (DVT), emergency department (ED) & Intensive Care Unit (ICU) admissions suggest a potential protective effect that could benefit populations without readily available access to SARS-CoV-2 vaccination. Thus further investigation with future prospective studies is warranted.
Conflict of interest statement
Dr. Holton serves as a consultant for Acelity/3M and Stryker. Dr. Slavin, Ms. Taghioff, and Dr. Singh have no relevant disclosures. The authors have not received any consulting fees, stock options, research funding, capital equipment, or educational grants from TriNetX.
Figures
References
-
- COVID-19 map—johns Hopkins Coronavirus resource Center. Jhu.edu. Accessed January 29, 2021. https://coronavirus.jhu.edu/map.html.
-
- TriNetX. Trinetx.com. Published May 18, 2018. Accessed January 29, 2021. https://www.trinetx.com.
-
- Singh S, Bilal M, Pakhchanian H, Raiker R, Kochhar GS, Thompson CC. Impact of obesity on outcomes of patients with coronavirus disease 2019 in the united states: A multicenter electronic health records network study. Gastroenterology. 2020;159(6):2221-2225.e6. doi: S0016-5085(20)35067-8 [pii]. doi: 10.1053/j.gastro.2020.08.028 - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

