Microbial assemblages and methanogenesis pathways impact methane production and foaming in manure deep-pit storages
- PMID: 34343206
- PMCID: PMC8330953
- DOI: 10.1371/journal.pone.0254730
Microbial assemblages and methanogenesis pathways impact methane production and foaming in manure deep-pit storages
Abstract
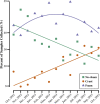
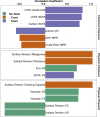
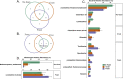
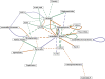
Foam accumulation in swine manure deep-pits has been linked to explosions and flash fires that pose devastating threats to humans and livestock. It is clear that methane accumulation within these pits is the fuel for the fire; it is not understood what microbial drivers cause the accumulation and stabilization of methane. Here, we conducted a 13-month field study to survey the physical, chemical, and biological changes of pit-manure across 46 farms in Iowa. Our results showed that an increased methane production rate was associated with less digestible feed ingredients, suggesting that diet influences the storage pit's microbiome. Targeted sequencing of the bacterial 16S rRNA and archaeal mcrA genes was used to identify microbial communities' role and influence. We found that microbial communities in foaming and non-foaming manure were significantly different, and that the bacterial communities of foaming manure were more stable than those of non-foaming manure. Foaming manure methanogen communities were enriched with uncharacterized methanogens whose presence strongly correlated with high methane production rates. We also observed strong correlations between feed ration, manure characteristics, and the relative abundance of specific taxa, suggesting that manure foaming is linked to microbial community assemblage driven by efficient free long-chain fatty acid degradation by hydrogenotrophic methanogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- Smil V. Worldwide transformation of diets, burdens of meat production and opportunities for novel food proteins. Enzyme and Microbial Technology. 2002. pp. 305–311. doi: 10.1016/S0141-0229(01)00504-X - DOI
-
- Jacobson LD, Hu B, Yan M, Akdeniz N, Clanton C. The potential causes of manure pit foaming in pig finishing barns. Biol Eng Trans. 2013;7004: 4764–4773.
-
- Foaming Manure. In: Ontario Ministry of Agriculture, Food and Rural Affairs [Internet]. 2016 [cited 9 Feb 2021]. Available: http://www.omafra.gov.on.ca/english/livestock/swine/news/mayjun10a1.htm
-
- Hoff SJ. Manure foaming issues. In: Professional Pig Community; [Internet]. 2015. [cited 9 Feb 2021]. Available: https://www.pig333.com/articles/manure-foaming-issues_10067/
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

