Cardiac allograft vasculopathy: current review and future research directions
- PMID: 34343276
- PMCID: PMC8783389
- DOI: 10.1093/cvr/cvab259
Cardiac allograft vasculopathy: current review and future research directions
Abstract
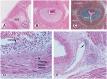
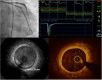
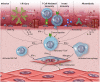
Cardiac allograft vasculopathy (CAV) is a pathologic immune-mediated remodelling of the vasculature in transplanted hearts and, by impairing perfusion, is the major cause of late graft loss. Although best understood following cardiac transplantation, similar forms of allograft vasculopathy occur in other vascularized organ grafts and some features of CAV may be shared with other immune-mediated vasculopathies. Here, we describe the incidence and diagnosis, the nature of the vascular remodelling, immune and non-immune contributions to pathogenesis, current therapies, and future areas of research in CAV.
Keywords: chronic rejection; endothelial cells; heart transplantation; innate and adaptive immunity; vascular smooth muscle cells.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2021. For permissions, please email: journals.permissions@oup.com.
Figures
References
-
- Bruno S, Remuzzi G, Ruggenenti P. Transplant renal artery stenosis. J Am Soc Nephrol 2004;15:134–141. - PubMed
-
- Kollar B, Kamat P, Klein HJ, Waldner M, Schweizer R, Plock JA. The significance of vascular alterations in acute and chronic rejection for vascularized composite allotransplantation. J Vasc Res 2019;56:163–180. - PubMed
-
- Harifi G, Nour-Eldine W, Noureldine MHA, Berjaoui MB, Kallas R, Khoury R, Uthman I, Al-Saleh J, Khamashta MA. Arterial stenosis in antiphospholipid syndrome: update on the unrevealed mechanisms of an endothelial disease. Autoimmun Rev 2018;17:256–266. - PubMed
-
- Khush KK, Cherikh WS, Chambers DC, Harhay MO, Hayes D Jr, Hsich E, Meiser B, Potena L, Robinson A, Rossano JW, Sadavarte A, Singh TP, Zuckermann A, Stehlik J; International Society for Heart and Lung Transplantation. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: thirty-sixth adult heart transplantation report - 2019; focus theme: donor and recipient size match. J Heart Lung Transplant 2019;38:1056–1066. - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

