Separable regulation of POW1 in grain size and leaf angle development in rice
- PMID: 34343399
- PMCID: PMC8633490
- DOI: 10.1111/pbi.13677
Separable regulation of POW1 in grain size and leaf angle development in rice
Abstract
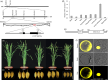
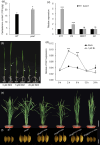
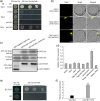
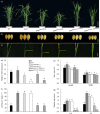
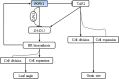
Leaf angle is one of the key factors that determines rice plant architecture. However, the improvement of leaf angle erectness is often accompanied by unfavourable changes in other traits, especially grain size reduction. In this study, we identified the pow1 (put on weight 1) mutant that leads to increased grain size and leaf angle, typical brassinosteroid (BR)-related phenotypes caused by excessive cell proliferation and cell expansion. We show that modulation of the BR biosynthesis genes OsDWARF4 (D4) and D11 and the BR signalling gene D61 could rescue the phenotype of leaf angle but not grain size in the pow1 mutant. We further demonstrated that POW1 functions in grain size regulation by repressing the transactivation activity of the interacting protein TAF2, a highly conserved member of the TFIID transcription initiation complex. Down-regulation of TAF2 rescued the enlarged grain size of pow1 but had little effect on the increased leaf angle phenotype of the mutant. The separable functions of the POW1-TAF2 and POW1-BR modules in grain size and leaf angle control provide a promising strategy for designing varieties with compact plant architecture and increased grain size, thus promoting high-yield breeding in rice.
Keywords: POW1; TAF2; brassinosteroid.; grain size; leaf angle; rice.
© 2021 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Ang, L.H. , Chattopadhyay, S. , Wei, N. , Oyama, T. , Okada, K. , Batschauer, A. and Deng, X.W. (1998) Molecular interaction between COP1 and HY5 defines a regulatory switch for light control of Arabidopsis development. Mol. Cell, 1, 213–222. - PubMed
-
- Asami, T. , Mizutani, M. , Fujioka, S. , Goda, H. , Min, Y.K. , Shimada, Y. , Nakano, T. et al. (2001) Selective interaction of triazole derivatives with DWF4, a cytochrome P450 monooxygenase of the brassinosteroid biosynthetic pathway, correlates with brassinosteroid deficiency in Planta. J. Biol. Chem. 276, 25687–25691. - PubMed
-
- Bahat, A. , Kedmi, R. , Gazit, K. , Richardo‐Lax, I. , Ainbinder, E. and Dikstein, R. (2013) TAF4b and TAF4 differentially regulate mouse embryonic stem cells maintenance and proliferation. Genes Cells, 18, 225–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

