Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2
- PMID: 34343897
- PMCID: PMC8299225
- DOI: 10.1016/j.biopha.2021.111953
Engineering of the current nucleoside-modified mRNA-LNP vaccines against SARS-CoV-2
Abstract
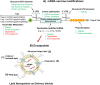
Currently, there are over 230 different COVID-19 vaccines under development around the world. At least three decades of scientific development in RNA biology, immunology, structural biology, genetic engineering, chemical modification, and nanoparticle technologies allowed the accelerated development of fully synthetic messenger RNA (mRNA)-based vaccines within less than a year since the first report of a SARS-CoV-2 infection. mRNA-based vaccines have been shown to elicit broadly protective immune responses, with the added advantage of being amenable to rapid and flexible manufacturing processes. This review recapitulates current advances in engineering the first two SARS-CoV-2-spike-encoding nucleoside-modified mRNA vaccines, highlighting the strategies followed to potentiate their effectiveness and safety, thus facilitating an agile response to the current COVID-19 pandemic.
Keywords: LNP, vaccines; MRNA; Nucleoside-modified; SARS-CoV-2; Spike protein.
Copyright © 2021 The Authors. Published by Elsevier Masson SAS.. All rights reserved.
Conflict of interest statement
None
Figures
References
-
- Wolff J.A., Malone R.W., Williams P., Chong W., Acsadi G., Jani A., Felgner P.L. Direct gene transfer into mouse muscle in vivo. Science. 1990;247(4949 Pt 1):1465–1468. - PubMed
-
- Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., McDermott A., Flach B., Doria-Rose N.A., Corbett K.S., Morabito K.M., O’Dell S., Schmidt S.D., Swanson P.A., 2nd, Padilla M., Mascola J.R., Neuzil K.M., Bennett H., Sun W., Peters E., Makowski M., Albert J., Cross K., Buchanan W., Pikaart-Tautges R., Ledgerwood J.E., Graham B.S., Beigel J.H. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383(20):1920–1931. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

