Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: A systematic review and meta-analysis of randomized controlled trials
- PMID: 34343937
- PMCID: PMC8324418
- DOI: 10.1016/j.intimp.2021.108027
Clinical efficacy and safety of Janus kinase inhibitors for COVID-19: A systematic review and meta-analysis of randomized controlled trials
Abstract
Objectives: This systematic review and meta-analysis of randomized controlled trials (RCTs) aimed to investigate the clinical efficacy and safety of Janus kinase (JAK) inhibitors for COVID-19 patients.
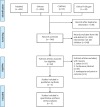
Methods: PubMed, Embase, Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov were searched from inception to July 12, 2021. RCTs comparing the clinical efficacy and safety of JAK inhibitors with a placebo or standard care in treating COVID-19 patients were included. The primary outcome was all-cause mortality rate at day 28.
Results: Three RCTs were included in this meta-analysis. The all-cause mortality rate at day 28 was lower among the patients receiving JAK inhibitors than among the controls (4.1% [28/647] versus 7.0% [48/684], OR, 0.57; 95% CI, 0.36-0.92, I2 = 0). The clinical recovery rate was higher among the patients receiving JAK inhibitors than among the controls (85.1% (579/680) versus 80.0% [547/684], OR, 1.45; 95% CI, 1.09-1.93, I2 = 0). Additionally, the use of JAK inhibitors was associated with a shorter time to recovery than among the controls (MD, -2.84; 95% CI, -5.56 to -0.12; I2 = 50%). The rate of invasive mechanical ventilation (MV) was lower in the patients who used JAK inhibitors than among the controls. Finally, no significant difference was observed between the patients who used JAK inhibitors and the controls in the risk of any adverse events (OR, 0.92; 95% CI, 0.64-1.34; I2 = 33%) and serious adverse events (OR, 0.80; 95% CI, 0.45-1.44; I2 = 46%).
Conclusions: JAK inhibitors can lead to a better clinical outcome of hospitalized COVID-19 patients, and they are a safe agent in the treatment of COVID-19.
Keywords: And tofacitinib; Baricitinib; COVID-19; Janus kinase inhibitor; Ruxolitinib.
Copyright © 2021 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures






References
-
- WHO. https://covid19.who.int/?gclid=CjwKCAjwiLGGBhAqEiwAgq3q_qP6pRDB-zQNmYa-d... Accessed on June 18, 2021.
-
- Berlin D.A., Gulick R.M., Martinez F.J. Severe Covid-19. N Engl J Med. 2020;383:2451–2460. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

