Exhaled breath biomarkers of influenza infection and influenza vaccination
- PMID: 34343985
- PMCID: PMC8763390
- DOI: 10.1088/1752-7163/ac1a61
Exhaled breath biomarkers of influenza infection and influenza vaccination
Abstract
Respiratory viral infections are considered a major public health threat, and breath metabolomics can provide new ways to detect and understand how specific viruses affect the human pulmonary system. In this pilot study, we characterized the metabolic composition of human breath for an early diagnosis and differentiation of influenza viral infection, as well as other types of upper respiratory viral infections. We first studied the non-specific effects of planned seasonal influenza vaccines on breath metabolites in healthy subjects after receiving the immunization. We then investigated changes in breath content from hospitalized patients with flu-like symptoms and confirmed upper respiratory viral infection. The exhaled breath was sampled using a custom-made breath condenser, and exhaled breath condensate (EBC) samples were analysed using liquid chromatography coupled to quadruplole-time-of-flight mass spectrometer (LC-qTOF). All metabolomic data was analysed using both targeted and untargeted approaches to detect specific known biomarkers from inflammatory and oxidative stress biomarkers, as well as new molecules associated with specific infections. We were able to find clear differences between breath samples collected before and after flu vaccine administration, together with potential biomarkers that are related to inflammatory processes and oxidative stress. Moreover, we were also able to discriminate samples from patients with flu-related symptoms that were diagnosed with confirmatory respiratory viral panels (RVPs). RVP positive and negative differences were identified, as well as differences between specific viruses defined. These results provide very promising information for the further study of the effect of influenza A and other viruses in human systems by using a simple and non-invasive specimen like breath.
Keywords: breath analysis; exhaled breath condensate (EBC); influenza; metabolomics; vaccine.
© 2021 IOP Publishing Ltd.
Figures
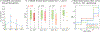
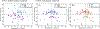
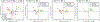
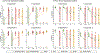
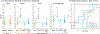
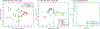
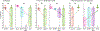
References
-
- Davis CE et al. 2020. Breath analysis for respiratory infections Breathborne Biomarkers and the Human Volatilome (Netherlands: Elsevier; ) pp 335–47
-
- CDC 2021. Disease Burden of Influenza (available at: www.cdc.gov/flu/about/burden/index.html) (Accessed 4 September 2021)
-
- Pawelec G and McElhaney J 2020. Recent advances in influenza vaccines F1000Research 9 F1000 Faculty Rev–305
