Positron emission tomography and single photon emission computed tomography imaging of tertiary lymphoid structures during the development of lupus nephritis
- PMID: 34344200
- PMCID: PMC8351034
- DOI: 10.1177/20587384211033683
Positron emission tomography and single photon emission computed tomography imaging of tertiary lymphoid structures during the development of lupus nephritis
Abstract
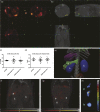
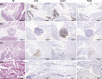
Lymphoid neogenesis occurs in tissues targeted by chronic inflammatory processes, such as infection and autoimmunity. In systemic lupus erythematosus (SLE), such structures develop within the kidneys of lupus-prone mice ((NZBXNZW)F1) and are observed in kidney biopsies taken from SLE patients with lupus nephritis (LN). The purpose of this prospective longitudinal animal study was to detect early kidney changes and tertiary lymphoid structures (TLS) using in vivo imaging. Positron emission tomography (PET) by tail vein injection of 18-F-fluoro-2-deoxy-D-glucose (18F-FDG)(PET/FDG) combined with computed tomography (CT) for anatomical localization and single photon emission computed tomography (SPECT) by intraperitoneal injection of 99mTC labeled Albumin Nanocoll (99mTC-Nanocoll) were performed on different disease stages of NZB/W mice (n = 40) and on aged matched control mice (BALB/c) (n = 20). By using one-way ANOVA analyses, we compared two different compartmental models for the quantitative measure of 18F-FDG uptake within the kidneys. Using a new five-compartment model, we observed that glomerular filtration of 18FFDG in lupus-prone mice decreased significantly by disease progression measured by anti-dsDNA Ab production and before onset of proteinuria. We could not visualize TLS within the kidneys, but we were able to visualize pancreatic TLS using 99mTC Nanocoll SPECT. Based on our findings, we conclude that the five-compartment model can be used to measure changes of FDG uptake within the kidney. However, new optimal PET/SPECT tracer administration sites together with more specific tracers in combination with magnetic resonance imaging (MRI) may make it possible to detect formation of TLS and LN before clinical manifestations.
Keywords: 18-F-fluoro-2-deoxy-D-glucose; 99mTC-Nanocoll; lupus nephritis; positron emission tomography; single photon emission computed tomography; systemic lupus erythematosus; tertiary lymphoid structures.
Conflict of interest statement
Figures



References
-
- Lahita R. (1999) The clinical presentation of systemic lupus erythematosus. In: Lahitha R. (ed) Systemic Lupsus Erythematosus. 3rd edition. San Diego, CA: Academic Press, 325–336.
-
- Balow J, Boumpas D, Austiniii H., III (2004) Systemic lupus erythematosus and the kidney. In: Lahita RG. (ed) Systemic Lupus Erythematosus. 4th edition. New York, NY: Elsevier academic press, 877–912.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

