Systematic review and meta-analysis: real-world data rates of deep remission with anti-TNFα in inflammatory bowel disease
- PMID: 34344314
- PMCID: PMC8335971
- DOI: 10.1186/s12876-021-01883-6
Systematic review and meta-analysis: real-world data rates of deep remission with anti-TNFα in inflammatory bowel disease
Abstract
Background: Deep remission (DR) is a treatment target in IBD associated with reduced hospitalization and improved outcome. Randomized control trial (RCT) data demonstrates efficacy of anti-TNFα agents in achieving DR; however, real-world data (RWD) can provide information complementary to RCTs, specifically regarding treatment duration. In this systematic review with meta-analysis, we use real-world data (RWD) to determine rates of DR in IBD treated with anti-TNFα.
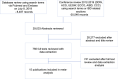
Methods: We completed a systematic search of MEDLINE and EMBASE on July 8, 2019 with review of major gastrointestinal conference abstracts from 2012 to 2019. Studies utilizing RWD (data not from phase I-III RCTs) of adult IBD patients treated with anti-TNFα agents were included. DR was defined by clinical and endoscopic remission at minimum. DR was assessed at 8 weeks, 6 months, 1 year, and 2 years. Risk of bias was assessed with the Newcastle Ottawa Scale.
Results: 29,033 publications were identified. Fifteen publications, nine manuscripts and six conference abstracts, were included encompassing 1212 patients (769 Crohn's disease-CD, 443 ulcerative colitis-UC), and analyzed using Comprehensive Meta-Analysis. Rate of DR was 36.4% (95% CI 12.6-69.4%) at 8 weeks, 39.1% (95% CI 10.4-78%) at 6 months, 44.4% (95% CI 34.6-54.6%) at 1 year, and 36% (95% CI 18.7-58%) at 2 years. DR in CD at 1 year was 48.6% (95% CI 32.8-64.7%) and in UC was 43.6% (95% CI 32.8-55.1%).
Conclusions: The rate of DR was highest after 1 year of therapy, in nearly 45% of IBD patients treated with anti-TNFα. Similar rates were achieved between patients with UC and CD. The findings highlight the efficacy of anti-TNFα in real-world setting. Future studies using RWD can determine efficacy of newer IBD therapeutics in routine clinical practice.
Keywords: Crohn’s disease; Inflammatory bowel disease; Remission; Tumor necrosis factor-a inhibitor; Ulcerative colitis.
© 2021. The Author(s).
Conflict of interest statement
The authors declare they have no competing interests.
Figures




Similar articles
-
Fecal transplantation for treatment of inflammatory bowel disease.Cochrane Database Syst Rev. 2018 Nov 13;11(11):CD012774. doi: 10.1002/14651858.CD012774.pub2. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2023 Apr 25;4:CD012774. doi: 10.1002/14651858.CD012774.pub3. PMID: 30480772 Free PMC article. Updated.
-
Effectiveness and safety of upadacitinib for inflammatory bowel disease: A systematic review and meta-analysis of RCT and real-world observational studies.Int Immunopharmacol. 2024 Jan 5;126:111229. doi: 10.1016/j.intimp.2023.111229. Epub 2023 Nov 16. Int Immunopharmacol. 2024. PMID: 37977068
-
Relapse From Deep Remission After Therapeutic De-escalation in Inflammatory Bowel Disease: A Systematic Review and Meta-analysis.J Crohns Colitis. 2020 Oct 5;14(10):1413-1423. doi: 10.1093/ecco-jcc/jjaa087. J Crohns Colitis. 2020. PMID: 32335670 Free PMC article.
-
Real-world clinical effectiveness and safety of vedolizumab and anti-tumor necrosis factor alpha treatment in ulcerative colitis and Crohn's disease patients: a German retrospective chart review.BMC Gastroenterol. 2020 Jul 8;20(1):211. doi: 10.1186/s12876-020-01332-w. BMC Gastroenterol. 2020. PMID: 32640990 Free PMC article.
-
Effectiveness and Safety of Ustekinumab in Inflammatory Bowel Disease: A Systematic Review and Meta-Analysis.Dig Dis Sci. 2022 Mar;67(3):1018-1035. doi: 10.1007/s10620-021-06932-4. Epub 2021 Mar 16. Dig Dis Sci. 2022. PMID: 33723700
Cited by
-
Sustained mucosal colonization and fecal metabolic dysfunction by Bacteroides associates with fecal microbial transplant failure in ulcerative colitis patients.Sci Rep. 2024 Aug 9;14(1):18558. doi: 10.1038/s41598-024-62463-8. Sci Rep. 2024. PMID: 39122767 Free PMC article.
-
Efficacy and Safety of the Anti-mucosal Addressin Cell Adhesion Molecule-1 Antibody Ontamalimab in Patients with Moderate-to-Severe Ulcerative Colitis or Crohn's Disease.J Crohns Colitis. 2024 May 31;18(5):708-719. doi: 10.1093/ecco-jcc/jjad199. J Crohns Colitis. 2024. PMID: 38096402 Free PMC article. Clinical Trial.
-
Bacillus velezensis inhibits azoxymethane/dextran sulphate sodium induced colitis associated colorectal cancer via the small molecule HeLM.J Gastrointest Oncol. 2025 Apr 30;16(2):470-484. doi: 10.21037/jgo-24-610. Epub 2025 Apr 27. J Gastrointest Oncol. 2025. PMID: 40386616 Free PMC article.
-
Lack of Benefit for Early Escalation to Advanced Therapies in Ulcerative Colitis: Critical Appraisal of Current Evidence.J Crohns Colitis. 2023 Dec 30;17(12):2002-2011. doi: 10.1093/ecco-jcc/jjad106. J Crohns Colitis. 2023. PMID: 37345930 Free PMC article. Review.
-
Precision medicine in inflammatory bowel disease: Individualizing the use of biologics and small molecule therapies.World J Gastroenterol. 2023 Mar 14;29(10):1539-1550. doi: 10.3748/wjg.v29.i10.1539. World J Gastroenterol. 2023. PMID: 36970587 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

